March 15, 2012 (Vol. 32, No. 6)
Public Health Concerns Mandate New Approaches to Combat Bacterial Pathogens
The vaccine market experienced significant growth over the last decade, with global revenues exceeding $24 billion in 2010, up from $7 billion in 2004. Major growth drivers were the increasing awareness of vaccine-preventable diseases as well as the introduction of new products. Within this growing market, glycoconjugate vaccines for the prevention of bacterial infections constitute 25% of total sales, or about $7 billion annually.
Despite the success of glycoconjugate vaccines, many challenges remain. Many pathogens important to public health lack vaccines. There have been several attempts to develop a vaccine against Staphylococcus aureus but all have failed so far. Pseudomonas aeruginosa also lacks a vaccine. Other serious pathogens missing vaccines include Neisseria meningitides group B (MEN B), the urinary tract pathogen extra-intestinal pathogenic Escherichia coli (ExPEC), and diarrheal pathogens Shigella sp., enterotoxigenic E. coli (ETEC), and Salmonella sp.
Along with the lack of vaccines, resistance development against currently available antibiotics continues to challenge public health professionals.
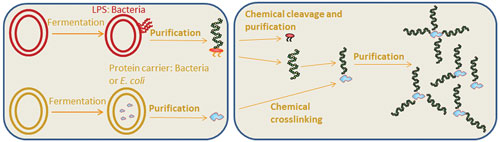
An important constraint on the potential for conjugate vaccines to prevent these infections is their complex chemistry-based manufacturing process (Figure 1). Manufacturing requires a multistep process of fermentation, purification, chemical processing, and cross-linking of the polysaccharide antigen with a bacterial protein carrier and a final purification step for the glycoprotein conjugate. Pathogenic organisms are required to provide the antigens, which can be challenging to manage.
The chemical conjugation process is non-specific so that there is heterogeneity in the final product and batch-to-batch variability occurs. Pharmaceutical companies have made significant strides in optimizing this process, and important vaccines are available against pathogens such as Streptococcus pneumoniae and MEN A and C. However, the chemistry-based process has not been able to tackle other important pathogens.
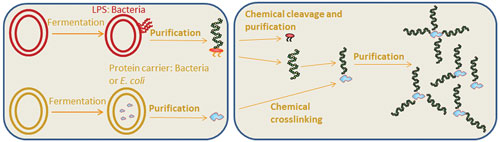
Figure 1. Bacterial conjugate manufacturing is a complex process that results in final product heterogeneity and batch-to-batch variability.
Unvaccined Pathogens
Conjugate vaccines are glycoprotein molecules (consisting of a protein and polysaccharide component linked together). These sugars are surface exposed bacterial antigens to which the body will develop an immune response. The protein carrier is responsible for eliciting a long-lasting immune response against the polysaccharide in young children, leading to protection against infection by the pathogen.
Contrary to the classical chemistry-based process, employing recombinant DNA technology enables the in vivo production of customized recombinant conjugate (bioconjugates) in E. coli (Figure 2). The platform allows the controlled design and production of glycoproteins with a customized polysaccharide structure, which will target bacterial pathogens that cannot be addressed with existing chemical processes.
Using such a well-understood and validated process has several advantages over chemistry-based vaccine development and manufacturing.
First, recombinant DNA technology injects considerable versatility into the vaccine development and manufacturing process. Because the expression of bacterial genes that code for specific polysaccharide and protein antigens can be controlled, almost any polysaccharide can be conjugated to almost any bacterial peptide. This enables the development of vaccine candidates against almost any bacterial pathogen of interest and it offers the opportunity to select for the most immunogenic components for the vaccine.
Second, the enzyme that links the polysaccharide to the protein is of bacterial origin as well. This allows the epitopes to be expressed in their most natural or native form, maximizing the immunogenicity of the complex, not only for the polysaccharide antigen but also for the protein carrier antigen enhancing the protection of the disease.
Finally, there is batch-to-bath consistency because the process is relatively straightforward and reproducible. This may minimize development and production costs as well as shorten the time to market launch.

Figure 2. Creation of a bacterial bioconjugate vaccine through recombinant DNA technology is relatively straightforward and generates consistent bioconjugate end product.
Clinical Trials
Although the recombinant DNA approach has many advantages over the chemistry approach, evidence is required to show that safe and immunogenic vaccines can be developed and that the manufacturing process is scalable if a vaccine candidate reaches the market. Although there is a significant amount of work still ahead before a vaccine candidate is licensed for sale, strong evidence is accumulating that recombinant DNA-based conjugate vaccine development and manufacturing is a valid approach and that pathogens without available vaccines today are feasible targets.
Philippe Dro ([email protected]) is CEO at GlycoVaxyn.