Historically, most therapeutics have been small chemicals and antibodies designed to target cellular proteins. New kinds of therapeutics are emerging, however, from a better understanding of noncoding RNAs (ncRNAs), such as the microRNAs (miRNAs) and short interfering RNAs (siRNAs), that participate in RNA interference (RNAi).
Now that RNAi pathways are being mapped, drug developers can exploit newfound mechanisms of action and realize pharamacologic windfalls.
Therapeutics that act on RNAi pathways not only circumvent many challenges related to protein druggability, they also demonstrate increased specificity and a decreased potential for adverse effects. As a result, RNAi-based therapies are being explored for a growing number of medical conditions.
“Over the last decade we have been leading the translation of RNAi technologies form bench to bedside,” asserts John Maraganore, Ph.D., CEO of Alnylam Pharmaceuticals. “And we are well on our way to bringing RNAi therapeutics forward as a new class of innovative medicines.”
One of the products in the Alnylam pipeline, Inclisiran, is an RNAi that targets PCSK9, a genetically validated regulator of the low-density lipoprotein (LDL) receptor. High PCSK9 levels have been associated with low levels of the LDL receptor and increased plasma LDL-cholesterol, which increases the risk of cardiovascular events. Inclisiran silences PCSK9 synthesis in hepatocytes and lowers LDL-cholesterol levels in patients with atherosclerotic cardiovascular disease.
Encouraging Findings
“Our Phase II study with Inclisiran is the largest study ever conducted with an RNAi therapeutic,” insists Dr. Maraganore. This study yielded results that were taken to confirm Inclisiran’s effectiveness. The drug led to a statistically significant decrease in LDL-cholesterol, of over 50%.
Another encouraging finding is that Inclisiran’s therapeutic effect is durable. The drug, Dr. Maraganore suggests, could be adminstered in quarterly or even biannual doses. Accordingly, the drug could help overcome one of the biggest challenges in hypercholesterolemic patients—compliance. Over half of these patients discontinue their cholesterol-lowering medications within one year after the first prescription.
“A drug like Inclisiran that can be given three to four times a year will be very welcome by the patient community,” predicts Dr. Maraganore. “An approach like this could fundamentally change the management of hypercholesterolemia.” For the Inclisiran program, Alnylam investigators are partnered with The Medicines Company.
Another therapeutic in the Alnylam pipeline, ALN-GO1, was developed to treat primary hyperoxaluria, a rare orphan disease in which excessive oxalate production causes the formation of kidney stones, leading to renal failure and other systemic effects. Pediatric patients typically have a poor clinical course, and the only treatment is double kidney and liver transplant.
“Our investigational RNAi therapeutic targets and silences an enzyme that is involved in oxalate metabolism,” details Dr. Maraganore. “This approach helps lower oxalate levels in patients.”
Investigators at Alnylam previously revealed that this drug can significantly elevate plasma and urinary glycolate in human volunteers in a dose-dependent manner, an effect that is consistent with its mechanism of action. More recently, two patients were enrolled into the ALN-GO1 program. Data are being expected by the end of the year.
“There are always ups and downs with drug development, and we have experienced them as well,” concludes Dr. Maraganore. “But with over 1,000 people that have been treated in our clinical studies, we feel encouraged about where we are today and about where this can go.”
Local Delivery
“Our product, siG12D-Loder, has completed Phase I and is about to enter Phase II clinical trials,” says Amotz Shemi, Ph.D, CEO and co-founder of Silenseed. Scientists at Silenseed developed Loder (local drug eluter), which the company describes as the first drug delivery platform that allows RNAi drugs to be directly delivered into solid tumors. Loder consists of a millimeter-scale biopolymeric scaffold matrix that contains an RNAi drug. After the matrix is inserted into the tumor core, Loder is released over a period of several months.
RNAi delivered by siG12D-Loder is designed to silence KRAS-harboring G12D, a mutation that generates an activated version of the protein. “The KRAS oncogene has so far been considered undruggable,” Dr. Shemi points out. KRAS G12D is a potent tumor initiator that was described in up to 95% of the pancreatic ductal adenocarcinomas.
An advantage of the Loder platform is that it delivers RNAi locally, within the tumor, circumventing systemic toxicity and the need for large systemically administered doses. “We have preclinical and clinical data showing that our treatment is safe,” asserts Dr. Shemi. “It affects the development of tumors and impedes the development of new metastases.”
Unpublished preclinical studies show that Loder may be used in combination with immuno-oncology treatments. “We strongly believe that this combination can open new markets for many companies for treating pancreatic cancer and possibly other solid tumors,” comments Dr. Shemi.
Many solid malignancies have immune-privileged microenvironments. As a result, immune cells cannot infiltrate them effectively. Researchers at Silenseed noticed that when RNAi is continually delivered into a tumor over several months, the tumor becomes much more permeable and possibly less immune privileged. “The most powerful treatment might be based on combinations,” suggests Dr. Shemi. “Combining RNAi and immuno-oncology is a huge challenge, but it can be very effective in solid tumors.”
Recent patents granted to Dr. Shemi and his colleagues at Silenseed cover therapeutics for additional oncogene targets. For example, there is an RNAi that targets the BMI-1 polycomb ring finger oncogene. This RNAi has been incorporated into a millimeter-scale biodegradable polymeric matrix drug-delivery device for the treatment of prostate cancer. “We will conduct preclinical studies in which this therapeutic will be delivered locally but for a long time, at least half a year,” notes Dr. Shemi.
Another research effort at Silenseed focuses on developing Loder-nano, a new-generation delivery platform for glioblastoma multiforme, the most frequent and most aggressive brain cancer. “We consider this second-generation Loder specifically, and not exclusively, for glioblastoma,” explains Dr. Shemi. “Unlike pancreatic cancer, the tumor is very diffuse, and there is a need to deliver the RNAi-based therapeutic to the entire brain.”
Lipid-Enabled and Unlocked
“One aspect that differentiates us from everyone else in the siRNA field is that we deliver our siRNA molecules with lipid-enabled and unlocked nucleic acid modified RNA (Lunar) technology,” says Joseph E. Payne, president and CEO of Arcturus Therapeutics. The Arcturus approach is to utilize an unlocked nucleomonomer agent (UNA) oligomer chemistry technology. According to the company, this technology can be used with different kinds of RNA, including messenger RNA (mRNA), miRNA, and siRNA. A critical distinguishing feature of the Lunar technology is its ability to deliver multiple siRNA molecules in one drug product.
An siRNA program for which Arcturus partnered with Johnson & Johnson uses three UNA oligomers in one drug product to target the hepatitis B virus. “Rather than having three separate injections for each molecule with a different pharmacokinetic profile, we have one drug product, one injection, that delivers simultaneously the three oligomers that attack the virus in a very rational way,” informs Mr. Payne.
The effective delivery of multiple RNA oligomers provides opportunities to apply this technology to other types of RNA-based medicines that require mixtures. For example, the technology could be effective with CRISPR/Cas9. With this approach, the Cas9-encoding mRNA and a guide strand RNA (which directs the gene-editing machinery to the appropriate gene) have to be delivered.
The Arcturus technology is distinct in another respect: it allows patients to receive injections containing much lower amounts of oligonucleotides. “Using the Lunar 2.0 technology, we can deliver RNA molecules subcutaneously, and our intravenous dosing is now in the 0.01–0.03 mg/kg range,” details Mr. Payne. With GalNac conjugate technology, much larger doses had to be administered, increasing the risk of adverse effects related to the formulations.
“In the past, the RNA pharma field has seen patients with serious diseases drop out of trials because of the associated injection site reactions,” recalls Mr. Payne. “But at doses less than 1 mg, which can be achieved with our Lunar 2.0 technology, the likelihood of injection site reactions attributed to the oligotherapeutic is very low.”
One of the preclinical programs at Arcturus is developing siRNA technologies for transthyretin amyloidosis. “We also have an siRNA program for hepatitis B, and undisclosed relationships with the pharmaceutical industry, one of which is for nonalcoholic steatohepatosis,” Mr. Payne points out. Nonalcoholic steatohepatosis is a liver disease characterized by inflammation, fibrosis, and cell death, and is estimated to affect 2–3% of the people in the U.S.
“Using our siRNA programs, we are going after very large patient population diseases and rare diseases,” declares Mr. Payne. Through the design and implementation of therapeutic approaches that propose to target large patient populations, Arcturus is addressing very specific obstacles that the RNAi field has to overcome. “We foresee exciting challenges with scale-up,” states Mr. Payne. “We will be exploring scales that have never been evaluated or achieved in the industry.”
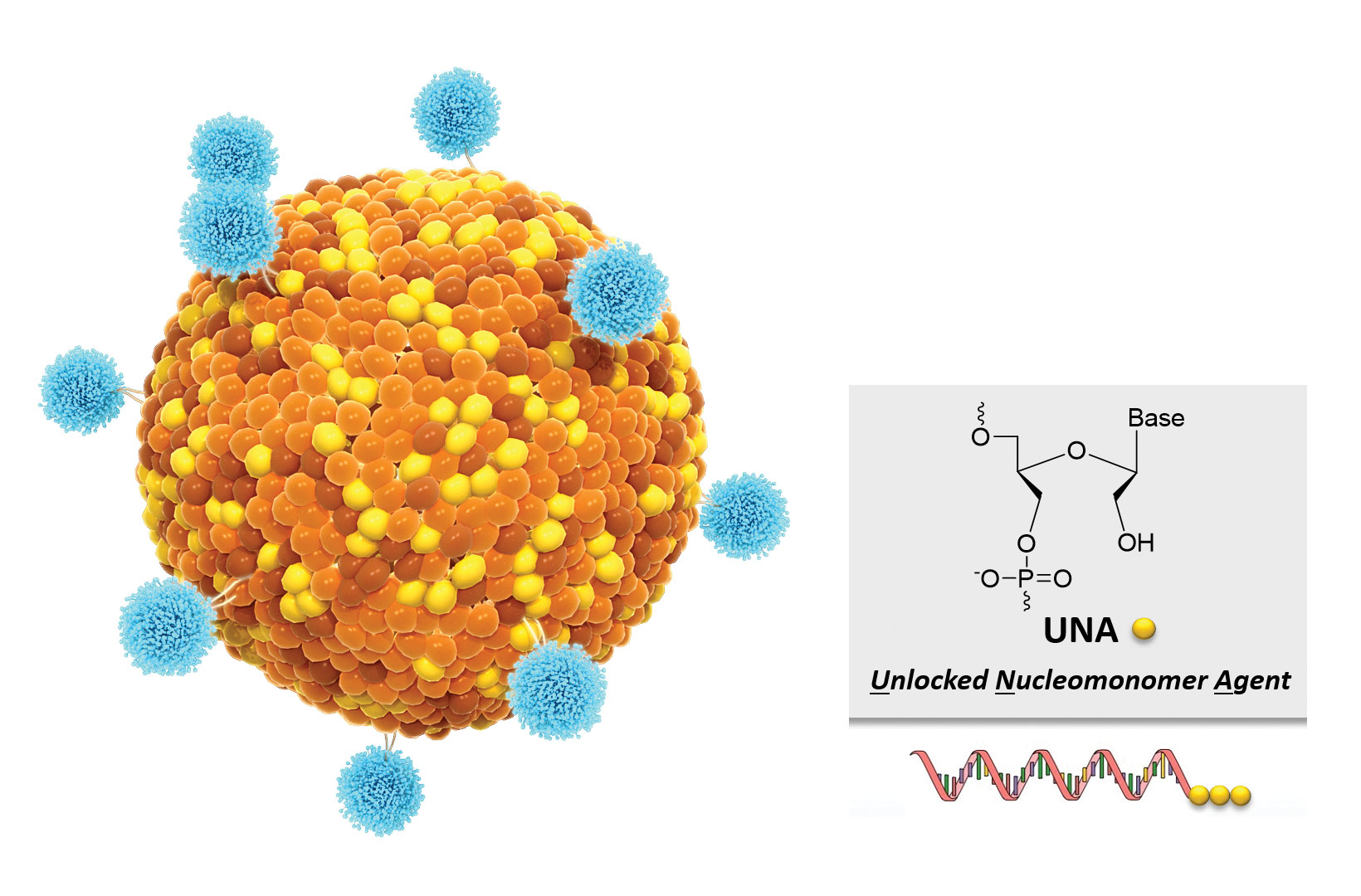
Arcturus Therapeutics develops novel RNA interference (RNAi) therapeutics by using its unlocked nucleomonomer agent (UNA) chemistry as well as its lipid-enabled and unlocked nucleomonomer agent RNA (LUNAR) nanoparticle delivery system. According to the company, UNA oligomers are more stable, more potent, and less toxic than standard oligomers, and LUNAR allows systemic delivery of all types of RNAi therapeutics to target tissues.
Vehicle-Free Delivery
“Our platform is somewhat different from others in the field,” confides Karen Bulock, Ph.D, vice president of research at RXi Pharmaceuticals. “Some of the chemical modifications that we use make sure that the RNAi molecules can enter the cells without any additional delivery vehicles.” The platform used by scientists at RXi Pharmaceuticals does not rely on a specific targeting method. “Therefore, the compounds that are delivered are not limited to any particularly cell type, but can be taken up by many different types of cells,” explains Dr. Bulock.
The first clinical candidate developed at RXi Pharmaceuticals was RXI-109, a self-delivering RNAi molecule that is administered by intradermal injection and targets connective tissue growth factor, which is involved in dermal scarring. “In a Phase II clinical trial, we are using this product after scar revision surgery to prevent the recurrence of hypertrophic scars,” informs Dr. Bulock.
One of the challenges with skin scarring is that once a hypertrophic scar forms, it tends to re-emerge at subsequent surgeries in the same individual. “We thought if we were to administer RXI-109 for several months after the revision surgery, while the scar is healing itself, we would have a better chance of preventing the scar from returning,” says Dr. Bulock.
Putting this notion to the test in a clinical trial, investigators at RXi Pharmaceuticals showed that using RXI-109 after scar revision surgery is beneficial. “Independent experts were asked to assign scores to treated and untreated scars,” notes Dr. Bulock. “About 65% of the time, the scores for the treated scars were better.” It is anticipated that the study will be completed this year.
Due to the involvement of connective tissue growth factor in healing and fibrosis in both the skin and the eye, investigators at RXi Pharmaceuticals are also testing RXI-109 in patients with age-related macular degeneration. “Because RXI-109 inhibits connective tissue growth factor, in an early trial we have been looking at whether we can prevent connective-tissue scar formation in the retina,” says Dr. Bulock.
In age-related macular degeneration, leaky blood vessels contribute to retinal degradation and eventual scarring. Although available treatments decrease the permeability of the blood vessels, scarring may still develop in up to half of the patients within two years.
In a Phase I/II trial, Dr. Bulock and colleagues looked at safety and efficacy endpoints with respect to differences in scar formation in the treated versus the untreated eye after RXI-109 administration by intraocular injection. “We are still enrolling subjects in the last cohort, but RXI-109 has been very well tolerated,” states Dr. Bullock. “We will have a readout toward the end of the year.”
A new research endeavor at RXi Pharmaceuticals has emerged after its acquisition of MirImmune, a company that has developed the RXi Pharmaceuticals technology for use with checkpoint targets in immunotherapy. In this approach, immune cells are initially treated ex vivo and subsequently used for oncology applications with the help of cell therapy.
“Using this platform, we can take immune cells from patients, treat them to silence immune suppression checkpoints, and then put them back into the patients to target cancer,” maintains Dr. Bulock. The platform is still at the preclinical stage, but according to Dr. Bulock, RXi Pharmaceuticals is placing “a lot of resources” into its development.
DNA-Directed RNAi
“Our company combines the mechanism of action of RNAi with gene-therapy delivery, resulting in the potential therapeutic application of gene silencing in a one-and-done treatment,” says David Suhy, Ph.D., CSO at Benitec Biopharma. The therapeutic pipeline at Benitec involves products for applications in oncology, infectious diseases, ocular diseases, and rare genetic diseases.
The most advanced product in the oncology program at Benitec involves an in-licensed compound that consists of a DNA vector that produces approximately a 40-nucleotide antisense single-stranded RNA with specificity against epidermal growth factor receptor (EGFR), a protein produced in about 80% of head and neck squamous cell carcinomas. Interestingly enough, the process that underlies this approach is not RNAi, but a related process termed post-transcriptional gene silencing (PTGS).
“For this product, we will start a Phase II or II/III clinical trial in early 2018,” anticipates Dr. Suhy. This project is being developed as part of a strategic collaboration with NantVentures, an investment group founded by Patrick Soon-Shiong, M.D., the cancer research pioneer and billionaire philanthropist. The compound has performed well in two early-stage clinical studies.
“We are very excited about the data that was generated from the early clinical studies with the EGFR-AS construct, and we look forward to progressing the compound through clinical development,” reports Dr. Suhy. Yet he recognizes that, comparatively, PTGS is relatively inefficient as compared to RNAi. “This is why most of our products focus on RNAi,” states Dr. Suhy. RNAi-based approaches are more efficient because the cellular machinery that processes short RNA duplexes is already in place and is being used for the endogenous miRNA pathway.
In a follow-up product that expands upon the clinical learning from the EGFR-AS approach, investigators at Benitec are targeting the same EGFR target with a DNA-directed RNAi construct that produces short hairpin RNA (shRNA) that eventually gets processed into siRNA. “The DNA-directed RNAi (ddRNAi) approach uses a DNA template,” expalins Dr. Suhy. “Once this template gets into the target cell, it uses the cell’s own transcriptional machinery to produce the therapeutic hairpins.” This product is currently in the discovery stage in the Benitec pipeline.
Historically, companies that use siRNA have presynthesized the nucleic acid in the laboratory so that it could subsequently be packaged for delivery into cells. “What makes Benitec different than most of its peers,” asserts Dr. Suhy, “is that we are not delivering double-stranded RNA (dsRNA) into the target tissues but recombinant DNA plasmids or constructs, and we do that by using well-validated viral vectors.”
Moreover, whereas siRNA delivery has to be administered every few weeks to maintain therapeutic effects, once the DNA enters a target cell, it produces the shRNA for the lifetime of the cell. “That can be months or more typically, years of therapeutic expression,” notes Dr. Suhy.
Investigators at Benitec are also harnessing the ddRNAi technology to develop a treatment for hepatitis B that is administered intravenously to humans. “This is compound is ultimately meant to be administered on top of current standards of care that are already on the market,” informs Dr. Suhy. The product involves three shRNA hairpins that target well-conserved sequences in the RNA produced from the hepatitis B viral genome. “What makes this product different,” points out Dr. Suhy, “is that it is a gene-therapy approach with a one-time administration.”
Scientists at Benitec recently completed a hepatitis C Phase I/IIa clinical trial that used a similar approach, and that was the first time when a viral vector was used to systemically administer RNAi directly into humans. “From a commercial perspective, we abandoned that product,” admits Dr. Suhy. “We are taking the clinical learnings from that experience to our hepatitis B product.”
The ocular program at Benitec pursues a treatment with RNAi to silence members of the VEGF family pathway, which can lead to age-related macular degeneration when overexpressed. “We developed a gene-therapy vector that is different in the way it delivers our therapeutic construct,” declares Dr. Suhy. He adds, however, that the vector may be administered conventionally.
Most gene therapy programs developed to treat ocular diseases inject the therapeutic product underneath the posterior part of the retina in a complex surgical procedure. “The gene therapy vector developed by Benitec is injected into the vitreous of the eye to deliver our construct,” says Dr. Suhy. “This is no different than how many of the drugs used to treat diseases like AMD are currently administered.”
Within the Benitec program on rare genetic diseases, efforts are focusing on developing a therapeutic to treat oculopharyngeal muscular dystrophy (OPMD), a rare autosomal-dominant genetic condition that impairs swallowing in the affected elderly patients and eventually leads to death from malnutrition or pneumonia. “In the RNAi approach that Benitec is developing, we knockout the mutant form of the gene that causes this disease,” details Dr. Suhy. “Then, from same vector, we express a healthy copy of the same gene.”

Light microscopy has been used to confirm that nanoparticles and viral vectors are effective vehicles for the delivery of RNAi therapeutics, such as small interfering RNAs and short hairpin RNAs. Many delivery vehicles lodge in the liver, but researchers are also interested in vehicles that can reach other tissues or accumulate in solid tumors. iStock/PointImages (©Zoran Zemeski)
Staying on Target
“Our conventional product lines use similar designs and algorithms,” comments Louise Baskin, senior product manager at Dharmacon, GE Healthcare. “But our On-Target-plus siRNA contains additional modifications that enhance specificity and reduce off-targets.” The patented dual-strand modification used for On-Target-plus includes sense-strand inactivation and an antisense seed-region modification.
One of the major drawbacks of RNAi technologies is the concomitant silencing of genes other than the intended targets. Off-target silencing precludes the identification of the specific phenotype caused by the silencing of the intended target. “The On-Target-plus modification is one of the larger leaps that we made in the field,” asserts Ms. Baskin. Dharmacon provides On-Target-plus products for silencing human, mouse, and rat gene targets.
Other products at Dharmacon are focusing on improving siRNA delivery. “In Accell siRNA, the siRNA itself is modified with chemical modifications during synthesis in a way that facilitates its uptake by nearly every cell type that we tried, without the need for transfection agents, electroporation, or other instrument-based delivery systems,” informs Ms. Baskin. Accell siRNA has shown highly effective siRNA-mediated gene silencing in several cell types, including primary neurons, which are particularly difficult to transfect.
One of the drawbacks of the Accell siRNA is that serum in the culture media may interfere with the uptake, so cells requiring high serum culture conditions may not be suitable for use with Accell siRNA. “We worked on many different cell types in-house during its development,” reports Ms. Baskin. “A large amount of work is currently happening with customers, as demonstrated by a strong publication record.”
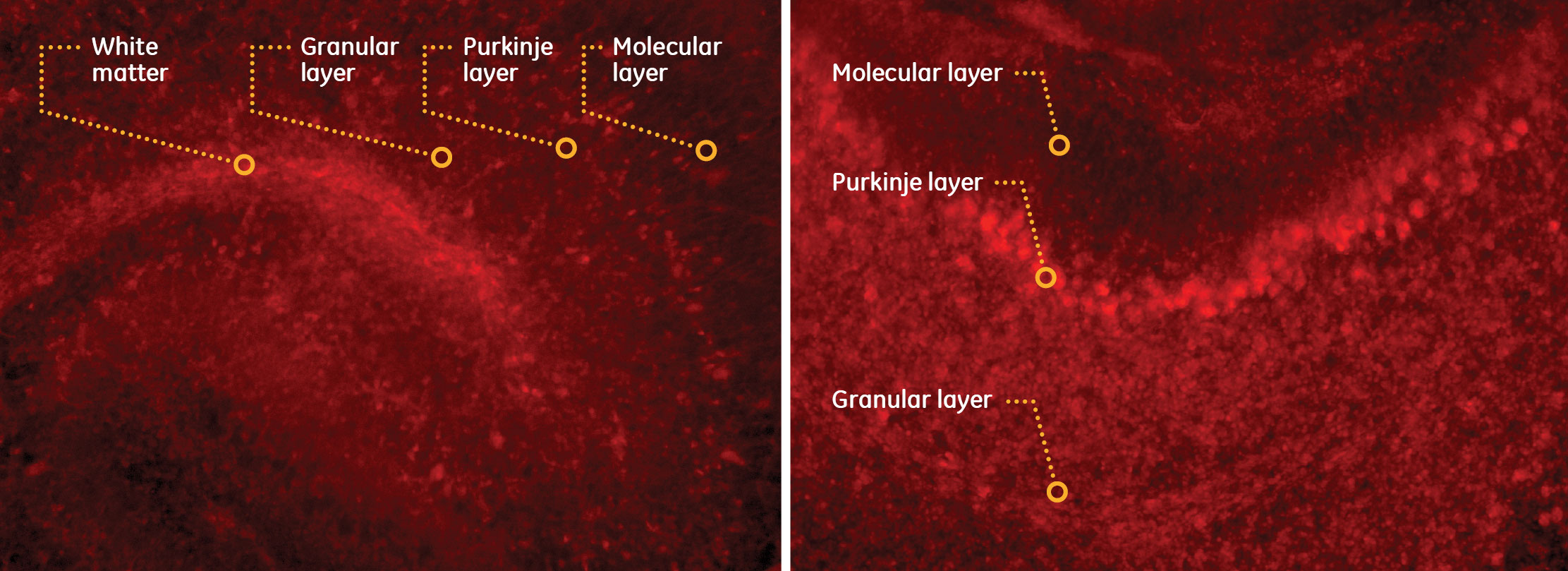
Short interfering RNA (siRNA) from Dharmacon, GE Healthcare, has shown effective gene silencing in several cell types. In these images, Dharmacon’s Red Non-targeting Control siRNA shows increased uptake in organotypic brain slice cultures where 250 µm cerebellar sections were prepared, cultured, and incubated for 3 hours (left) and 72 hours (right) prior to inspection by microscopy.


