June 1, 2010 (Vol. 30, No. 11)
Readily Detectable Agents Are Suitable for Presymptomatic and Early Diagnosis of Disease
Our increasing knowledge of the complex nature of molecular interactions has enabled us to not only understand physiological and pathological processes better but also to identify biological markers that define a particular state or condition—so-called biomarkers.
Molecular biomarkers are now used across many disciplines and can be any molecule, part of a molecule, or even a particular configuration that is both detectable and measurable, and the level or appearance of which is indicative of a particular biological state.
Biomarkers are useful tools in the diagnosis of disease or the identification of a predisposition. However, basing a clinical decision on a single biomarker can lead to a significant number of false positives. It is therefore more reliable and robust to use a panel of biomarkers that act as a fingerprint of the disease and its status.
Oxford Gene Technology (OGT) has experience in this area, and in this article we look specifically at the development of clinically relevant autoantibody-based diagnostic biomarker panels.
It is estimated that the biomarker market will be worth over $20 billion by 2014, driven by increasing demand from both the drug discovery and the clinical service sectors. Within the clinical sector, biomarkers impact multiple application areas, including diagnostics, prognostics, and companion diagnostics/personalized medicine.
Focusing on Diagnosis
Creating practical and robust diagnostic biomarker panels requires a dependable system for detecting and measuring them. Antibodies have several properties that make them excellent indicators of disease, and their detection forms the basis of many in vitro diagnostic tests.
Interestingly, in addition to generating antibodies against foreign molecules, the immune system also produces autoantibodies in response to a large number of pathogenic processes. The appearance of autoantibodies can precede disease symptoms by many years and, due to the inherent amplification of the immune system, they are readily detectable, making them ideal for presymptomatic and early diagnosis of disease.
When evaluating clinically relevant diagnostic tests, it is preferable to use those requiring the least invasive sample collection methods such as peripheral blood collection. Autoantibodies can be readily detected in blood serum.
Platform
OGT’s protein biomarker platform, developed through its Sense Proteomic subsidiary, enables detection of circulating serum autoantibodies using a functional protein microarray technology. Binding of autoantibodies in patient serum to proteins on the arrays is used to identify a subset of protein antigens that provides a characteristic fingerprint for a given disease.
In our discovery studies, clinically relevant autoantibodies and their cognate antigens were identified and characterized to formulate a panel of antigens, which provided specific diagnostic information for the particular disease. This is then followed by validation of the panel using additional patient samples and development of a diagnostic test with clinical utility.
Sense Proteomic has developed an array with ~1,300, folded, functional human proteins. The proteins were selected on the basis of important cellular processes or disease association and have clinical relevance to cancer and autoimmune disease. The proteins are individually expressed as recombinant fusion proteins with a biotin-carboxyl carrier protein (BCCP) tag that is biotinylated in the host cell only when the target protein and tag are correctly folded (Figure 1). This enables specific, on-array affinity purification of folded proteins using streptavidin-coated slide chemistry.
Many autoantibody reactive epitopes are conformation-dependent structures (i.e., discontinuous epitopes) that may not be detectable using peptides or proteins that are not in the correct conformation. Sense’s technology ensures that only fully folded proteins with native epitopes presented are immobilized on the array surface, thereby offering maximum likelihood of discovering multiple clinically relevant autoantibodies in a single assay (Figure 2).
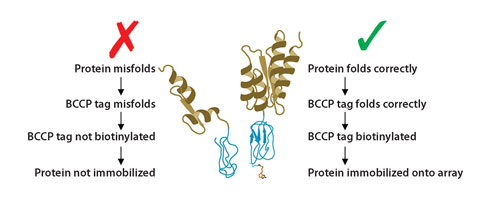
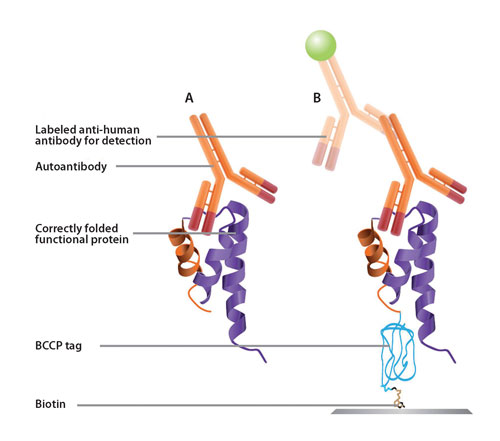
Figure 1. BCCP fusion tags ensure that only correctly folded proteins are immobilized.
Development
Sense Proteomic has developed diagnostic biomarker panels for prostate cancer and systemic lupus erythematosus using its functional protein array technology.
Screening for prostate cancer is generally performed by digital rectal examination and measurement of blood prostate specific antigen (PSA) titers. Men with elevated levels of PSA are usually referred for a prostate biopsy, but the majority of the biopsies prove to be negative. In practice, the PSA cut-off point of 4 ng/mL is considered sensitive but relatively nonspecific (86% sensitivity and 33% specificity), and there is a need for a diagnostic test with greater discriminatory power to assist in the early diagnosis of prostate cancer and reduce unnecessary treatments.
Using Sense Proteomic’s functional protein array technology, serum samples were assayed against more than 900 selected recombinant proteins. Advanced data analysis methods identified biomarkers that successfully distinguished prostate cancer from control samples. These were validated by two independent permutation assays that confirmed that the chosen biomarkers were related to the disease status of the sera.
The set of biomarkers identified were able to distinguish prostate cancer from control samples with both sensitivity and specificity above 90%. Further development and validation of this panel is continuing using a larger cohort of patient samples.

Figure 2. Sense Proteomic’s functional protein technology ensures maximum specificity and sensitivity of biomarker detection. (A) In vivo, autoantibodies bind to complex 3-D epitopes on auto-antigens. (B) On the Sense discovery array, the BCCP tag ensures that only correctly folded, functional recombinant proteins are biotinylated and present. This is vital for correct presentation of complex native epitopes. Correctly folded, functional proteins ensure maximum sensitivity and specificity of autoantibody biomarker detection.
Conclusion
Biomarkers must be both easy to detect and measure if they are to be used clinically. In addition, the use of a panel of biomarkers as opposed to a single biomarker can offer more robust and accurate results while minimizing the risk of false positives.
As such, circulating autoantibodies represent a pertinent source of early diagnostic and presymptomatic biomarkers for a number of pathologies including certain cancers and autoimmune diseases.
OGT has experience in the development of such panels using a number of different technologies. Core to the autoantibody area is Sense Proteomic’s functional protein array technology. This uses a BCCP fusion tag to provide the assurance that immobilized proteins are properly folded and hence native conformational epitopes are presented on the surface of each protein. As a result, the diagnostic autoantibody biomarker panels developed form sensitive and highly selective pathology fingerprints.
Michael McAndrew, Ph.D. ([email protected]), is head of molecular biology, Colin Wheeler, Ph.D., is head of assay development, and Rachel Fallon is director at Sense Proteomic. Web: www.senseproteomic.com. John Anson, Ph.D., is vp of biomarker discovery at Oxford Gene Technology.