G protein-coupled receptors (GPCR) are a large and diverse class of plasma membrane receptors involved in transduction of extracellular signals. The range of GPCR ligands is broad and includes small biogenic amines and peptides (e.g. cytokines) that bind to the extracellular domains of GPCRs to activate various signaling pathways. As GPCR ligands do not need to enter target cells, this receptor group is one of the most addressed family of therapeutic drug targets.
The Covalys (www.covalys.com) SNAP-tag is a protein tag that specifically forms a covalent thioether bond with fluorophores or other chemical entities presented as SNAP-tag substrates. In particular, this technology allows researchers to perform selective and on-demand labeling of SNAP-tag fusion proteins in living cells using a fluorophore of their choice. Labeling is routinely achieved by incubating living cells expressing a SNAP-tag fusion protein with a Benzylguanine (BG)-based fluorescent substrate for several minutes. Depending on the application, fluorescent BG-substrates can be selected to be plasma membrane permeable or impermeable.
Evaluation of SNAP-tag
The application of selectively labeling SNAP-tag-GPCRs to follow ligand-induced receptor internalization was investigated. The use of the SNAP-tag allows researchers to selectively label the specific pool of GPCRs present at the plasma membrane without labeling the internal pools.
To perform this study, the SNAP-tag containing an endoplasmic reticulum import sequence was fused to the N-terminus of various GPCRs (Sig(ER)-SNAP-GPCR). As a consequence, when the SNAP-GPCR fusion protein is inserted into the plasma membrane, the SNAP-tag is exposed to the extracellular environment.
Thus a noncell-permeable SNAP-tag substrate will label only the plasma-membrane-bound SNAP-GPCR proteins.
This selective labeling approach is expected to reduce intracellular background and thus improve the quality of images obtained by microscopic examination of cells.
This holds true for confocal microscopy studies, in which cross sections of cells will be virtually free from signals caused by labeled proteins and also for nonconfocal images where the homogeneous distribution of cell membrane proteins will change to the speckled pattern created by endocytotic vesicles.
The following GPCR model systems were used to investigate the selective labeling of SNAP-GPCRs followed by ligand induced receptor internalization: Neurokinin-1 Receptor and Endothelin-1 Receptor (Gq coupled), b2 Adrenergic Receptor (Gs coupled), and Chemokine Receptor 4 (Gi/Go coupled).
To perform these analysis, cells were transiently transfected by SNAP-tag plasmids using FuGene™ (Roche) according to the manufacturer’s instructions to express the receptor fusion proteins. Fifteen to 24 hours past transfection cells were labeled with the noncell-permeable SNAP-tag substrate BG-488 (fluorescein filter set compatible) at a concentration of 5 µM for 10 min at 37°C in complete medium. Excess substrate was washed away. Subsequently, the appropriate GPCR ligand was added in complete medium and incubated on the cells.
A significant change in the receptor distribution could be observed in all cases within 20 minutes after the start of incubation. Depending on the requirements, the SNAP-tag substrate can be replaced by one, e.g. at 547 nm or at 647 nm excitation wavelength.
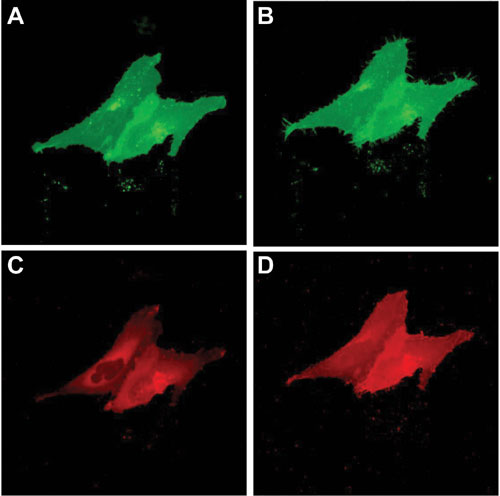
Figure 1 shows results for cells transfected with the SNAP-Endothelin-1 Receptor (all images were acquired by nonconfocal fluorescence microscopy). In resting cells, the receptor is homogeneously distributed in the plasma membrane (Figure 1A). Few bright spots can be detected in the cytoplasm indicating low internalization before stimulation.
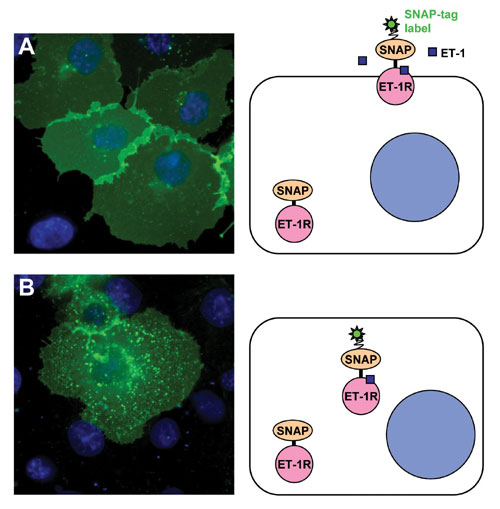
Post stimulation with endothelin-1 (Figure 1B), the receptor is massively relocalized to endocytic vesicles. Relocalization was clearly detectable at times post stimulation of 10 min at 37°C. Next to the micrographs a drawing depicts the situation before and after stimulation. Only SNAP-tag receptor labeled with noncell-permeable substrate contributes to the relocation observed in that experiment.
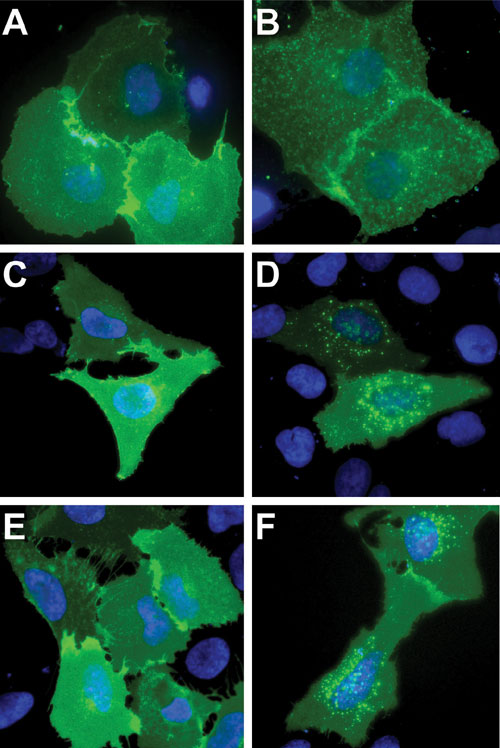
SNAP-tag fusions to Neurokinin-1 Receptor (ligand Substance P; expressed in COS-7 cells), Chemokine Receptor 4 (ligand SDF-1a; expressed in COS-7 cells), and b2-Adrenergic Receptor (ligand isoproterenol; expressed in U2-OS cells) also localized correctly to the plasma membrane and were able to internalize into endocytotic vesicles upon ligand stimulation (Figure 2).
As a control, SNAP-GPI, a fusion of SNAP-tag with a protein sequence that leads to the export of the protein to the outer leaflet of the cell membrane followed by transfer of its C-terminus to a glycophosphatidyl inositol anchor, was expressed. Homogeneous plasma membrane labeling was obtained after incubation with BG-488 substrate. Neither in the resting state nor after treatent with any of the ligands used for the GPCRs could any internalization into endosomal vesicles be detected, revealing that ligand-induced endocytosis was specific to binding of the appropriate ligand to its receptor.
Disruption of Functionality Test
To check whether the functionality of GPCRs was disturbed by the fusion to the SNAP-tag, PCKg was used as a reporter for GPCR activation. This protein relocates quickly from the cytosol to the plasma membrane upon activation.
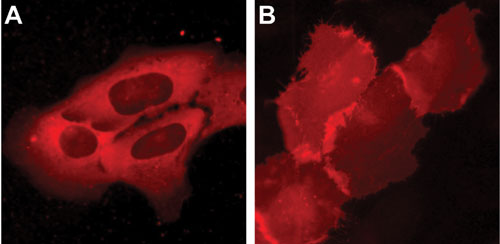
Functionality of PCKg-SNAP was tested successfully by incubation of the cells with a cell membrane permeable phorbol ester (TPA) that directly activates PCKg (Figure 3). Upon application of the respective GPCR-ligand, the same PCKg-SNAP relocation from the cytosol to the plasma membrane could be demonstrated.
Ligand stimulation of Gq-coupled GPCRs results in IP3 production followed by an elevation of cytosolic Ca2+ released from the endoplasmic reticulum. The increase in intracellular calcium activates PCKg.
Two Gq-coupled receptors, Neurokinin-1 Receptor and Endothelin-1 Receptor, fused to the SNAP-tag were used in these experiments. Cells were co-transfected with plasmids encoding for SNAP-GPCR and PCKg -SNAP fusion proteins. Cells were first incubated with the noncell-permeable substrate BG-488 to label the pool of SNAP-GPCR present at the cell surface.
Subsequently cells were treated with the cell-permeable SNAP-tag substrate TMR-Star to label the intracellular pool of PCKg-SNAP (Note: simultaneous labeling of the internal pool of SNAP-tag-receptor fusion protein results in background but did not prevent detection of a translocation of the PCKg).
Within two minutes, a rapid relocation of PCKg from the cytosol to the cell membrane was observed (Figure 4). Control experiments performed with Gs-coupled SNAP-b2 Adrenergic Receptor and SNAP-GPI show no detectable PCKg-SNAP relocation. This finding demonstrates that GPCRs modified with SNAP-tag at their N-terminus are fully functional and can propagate signals downstream from the plasma membrane.
Summary
SNAP-tag is an effective tool for selective and covalent site-specific labeling of GPCRs. The ability to exclusively label receptors at the cell surface without background from internal pools of labeled proteins associated with the use of autofluorescent proteins presents an attractive technology to perform receptor internalization assays. When fused to SNAP-tag, Gq-coupled GPCRs Neurokinin-1 Receptor and Endothelin-1 Receptor not only retained their ability to bind to their ligands but also internalized into endocytotic vesicles as described for the wildtype proteins. Ligand binding was shown to stimulate downstream signaling resulting in an increase in intracellular Ca2+ that could be monitored using PCKg as a reporter for Neurokinin-1 Receptor and Endothelin-1 Receptor.
Additional important topics are the legal and license situation for use of SNAP-tag labeling kits. These are supplied with an implicit license allowing straightforward commercial use. This avoids licensing of a fluorescent protein for assay development or for its application in HTS. The use of SNAP-tag for protein labeling followed by internalization or relocation assays will not infringe on patents granted on changes in the cellular distribution of proteins labeled with autofluorescent protein tags.
Andreas Brecht, Ph.D. ([email protected]), is CEO, Olivier Roumanie, Ph.D., is a staff scientist, and Kathrin Muntener, Ph.D., is an application scientist at Covalys.