Moving drug candidates through the various phases of development is a complex process. It is necessary to analyze the drug’s effect on the whole cell rather than separate, individual constituents.
The Bionas® 2500 analyzing system (Figure 1) provides continuous, quantitative, and long-term information concerning the biological status of cells. Changes in metabolism are measured automatically and in real time providing a biochemical fingerprint of the cellular physiology. Regeneration effects can be observed due to Bionas’ (www.bionas.de) perfusion system.

Bionas 2500 Analyzing System
The Bionas 2500 allows label-free and noninvasive monitoring of cellular physiology. It measures metabolically relevant parameters such as oxygen consumption, extracellular acidification rate, and surface adhesion (cell impedance/resistance) of test cells. The readout is continuous for up to several days.
The system allows parallel analysis of six sensor chips on which cells have been cultivated. It has an automatic fluidic element that supplies the cells with fresh medium or medium containing the test substance at regular, programmable intervals. It is equipped with control and evaluation software that permits direct control of the system and recorded data to be monitored online. The autosampler allows automatic and unattended operation of the measurement sequence with long walk-away times.
Continuous Monitoring Provides Deeper Insight
Bionas 2500 can be used with many different cell types including cell lines, primary cell cultures, tissue slices, and biopsies to investigate drug candidates or to validate targets. Applications include elucidating the mechanism of action of a substance, validating targets, or conducting toxicity studies.
A better understanding of cellular physiology is possible through dynamic monitoring with this system than with any other technologies available today. Continuous measurements also allow the monitoring of distinct fluctuations in acidification rate, oxygen consumption, and cellular adhesion over an extended time period. In comparison, endpoint-based methods such as protein assays are inherently not capable of detecting these fluctuations.
The Bionas system also provides a physiological fingerprint of what happens inside the cells because of its unbiased set-up. Combining the results with those from other techniques “helps us to move forward,” says Eric Ronken, Ph.D., principal scientist at Solvay Pharmaceuticals (www.solvaypharmaceuticals.com). Solvay uses the Bionas system for target validation.
“Sometimes the most cited signal transduction pathways may not be the relevant ones. For example, cAMP is regulated by many receptors, yet sometimes we see that certain kinases are components of the true predictive signaling route of a specific neural circuit.”
With the Bionas 2500 system, Dr. Ronken observes a receptor-mediated uncoupling of acidification and oxygen consumption. “Using combinations of agonists and antagonists, in the absence or presence of certain inhibitors, we can get a nice fingerprint of signaling cascades. Furthermore, the resistance parameter responds in its own unique way, delivering yet another parameter that may be of relevance in distinct brain areas, the pituitary gland, or indeed in any other cell or tissue compartment that may respond to specific stimuli.”
Predicting Hepatotoxicity through Parallel Studies in Human Cells
More than 30% of clinical projects fail because of toxicity issues. Therefore, stringent assays that allow prediction of human toxicity earlier in the drug development process are desperately needed. Although rodent cells are widely used in preclinical drug development, studies employing the Bionas system indicate that results based only on rodent cells may be misleading. Instead complementary studies in human cells could provide more meaningful results allowing more informed decisions for selecting the most promising substances for development.
Primary human hepatocytes, primary rat hepatocytes, and the human liver carcinoma cell line HepG2 were monitored in the presence of acetaminophen (APAP), which is known for its liver toxicity. The cells were seeded directly on the silicon surface of the sensor chips. The primary human hepatocytes were cultured using Primacyt’s (www.primacyt.com) HEPAC2 cell cultivation method.
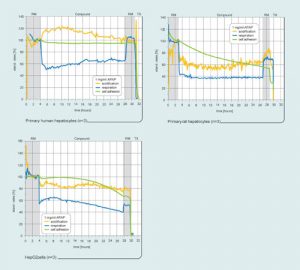
Under these conditions functional hepatocytes can be cultured for several weeks. In combination with the continuous process monitoring of the Bionas system, this provides, for the first time, an environment that is similar to the in vivo setting and thus helps scientists to elucidate the toxicity of drug candidates.
Figure 2 shows an experiment that begins with the running medium, which is without APAP. After three to four hours, 1 mg/mL APAP was added. While only the respiratory activity was reduced in human hepatocytes, the rat hepatocytes clearly showed stronger effects.
Elke Thedinga, Ph.D., is manager of R&D, and Michael Schulze, Ph.D. ([email protected]), is managing director at Bionas.






