December 1, 2012 (Vol. 32, No. 21)
Steven Blakely
Single-Molecule Counting Sheds Light on Chronic Disease Pathways and Progression
Dysregulation of the cytokine system has been implicated for many autoimmune and inflammatory disorders, such as rheumatoid arthritis, asthma, psoriasis and cardiovascular disease. Recent research has shown that detection and monitoring of cytokine concentrations can provide insights into disease progression and thus, cytokines have become attractive biomarkers and candidates for targeted therapies. For example, IL-17 biomarkers are key members of the TH-17 signaling pathway, serving to induce and mediate pro-inflammatory responses in numerous immune mediated diseases. However, due to the low-abundance of these circulating markers, such as IL-17A, obtaining robust measurements in clinical samples has been difficult.
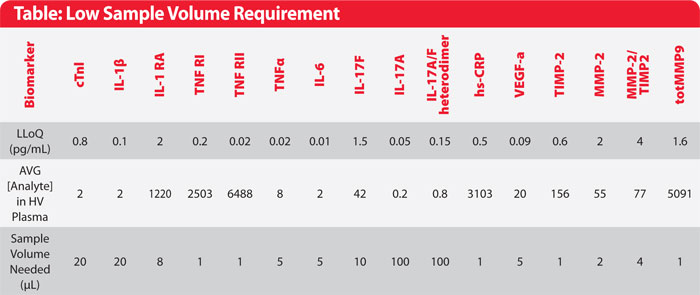
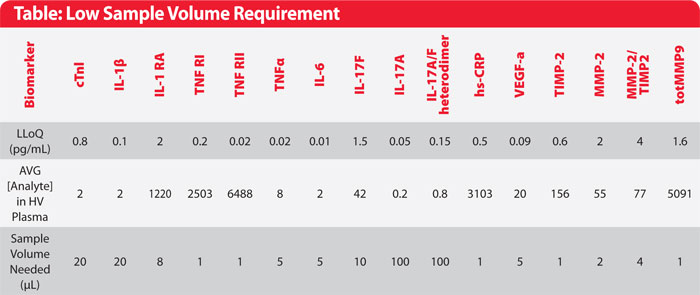
Table. Analytical performance of assays presented in this study. Singulex assays offer greater sensitivity and lower sample volume requirement, allowing baseline biomarker levels to be accurately measured in age-gender matched healthy volunteers.
To address this critical need, Singulex employs a proprietary single-molecule counting technology with increased detection sensitivity to help shed light on biomarker verification and validation programs. The patented Erenna® Immunoassay system, which includes uniquely optimized immunoassays, offers sub-picogram per mL resolution at an improvement of 1–3 fold over traditional immunoassays, and with a dynamic range of up to 4-logs. This sensitivity improvement helps minimize undetectable samples that could otherwise delay or derail clinical studies and enables biomarker quantification using minimal sample volume (as little as 1uL), allowing for conservation of precious clinical specimens (Table). Here we present case studies demonstrating how the use of the Erenna Immunoassay System has provided critical insights toward improving the clinical utility of biomarkers and accelerating the development of novel therapies for treating inflammatory diseases (Figures 1 and 2).
Figure 1. Case Study: RA vs. Healthy. A multimarker analysis of rheumatoid arthritis patients (RA) vs. age-gender matched healthy volunteers (HV) was conducted using the Singulex technology. The study identified markers with significant concentration differences between healthy and disease populations using a limited population set. In particular, Singulex solutions provide the sensitivity and quantifiability needed to accurately measure baseline IL-17A concentrations in HV, as well as minute elevations in RA. In conjunction with data from IL-17F, this dual marker differentiation powered by Singulex allows for 100% specificity for RA patients in this study.
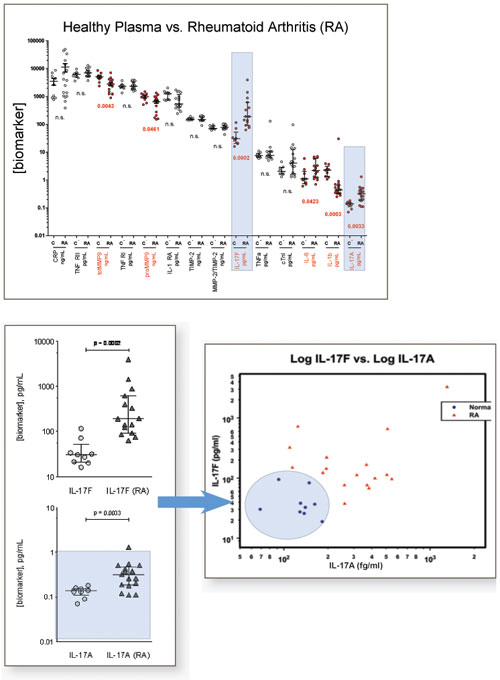
Figure 1
Figure 2. Case Study: RA vs. OA. A panel of inflammatory biomarkers was examined in DMARD (Disease Modifying Anti rheumatic Drugs)-naïve RA vs. knee OA (Osteoarthritis) patient cohorts (samples provided by Dr. Jeff Greenberg, NYU). (A) Markers that exhibited significant differences in plasma concentrations between the two cohorts included CRP, IL-6R alpha, IL-6, IL-1 RA, VEGF, TNF-RII, and (B) IL-17A, IL-17F, and IL-17A/F. Among the three tested isoforms of IL-17, the magnitude of elevation for IL-17F in RA patients was the highest. This data has been previously presented at the American College of Rheumatology 2011 by Dr. Greenberg’s group.

Figure 2
Digital Single-Molecule Counting Technology
Singulex high-definition immunoassays, coupled with the high-precision ultrasensitive Erenna Immunoassay System and proprietary Sgx link™ software, accelerate biomarker research by providing sub-picogram/mL biomarker detection across multiple disease areas.

Singulex Erenna Immunoassay system utilizes proprietary digital single-molecule counting technology to enable quantification of low abundant biomarkers using minimal sample volumes.
Conclusions
Previously available detection methods for quantifying low-abundance biomarkers, such as IL-17A, IL-17F, and IL-17AF were inadequate due to lower limits of quantification.
The patented single-molecule counting technology provided by Singulex offers superior sensitivity over traditional immunoassays. With high precision and sub-picogram per mL lower limits of quantification, only a minute sample volume is required for the determination of biomarker concentration at baseline levels. This allows precious sample to be conserved from high-profile clinical study specimens, and multipanel studies to be performed.
Singulex solutions provide high-resolution monitoring of baseline IL-17A concentrations that are present at low levels, allowing for a clear differentiation of the disease state from the healthy population. The technology also enabled quantification of other IL-17 isoforms in RA patients, which have not been well characterized before.
Singulex
Steven Blakely
Marketing Director
Acknowledgement: Singulex would like to thank the Greenberg Lab at NYU School of Medicine for their collaboration on studies presented.