Julia Baek Ph.D. Research Scientist Thermofisher Scientific
Jim Thayer Ph.D. Staff Research Biochemist Thermo Fisher Scientific
Ilze Birznieks Global Product Manager Thermo Fisher Scientific
This is the second article in this series discussing HPLC oligonucleotide separations. This article will discuss the separation of oligonucleotides and dsDNAs using a wide-pore, polymer based reversed-phase column.
Synthetic oligonucleotides are used extensively in laboratories as primers for polymerase chain reactions (PCR) and DNA sequencing, probes to visualize a specific DNA or RNA, tools to study gene function, and biopharmaceutical drugs for treating various diseases. Analyses of synthetic oligonucleotides are most commonly performed using ion-pair reversed-phase chromatography (IP-RP). IP-RP utilizes the ionic interaction between the analyte and the ion-pair reagent, which also interacts with the stationary phase via its hydrophobic moiety. IP-RP provides high-resolution separation of failure sequences and can be directly coupled to mass spectrometry for identification of target oligonucleotide and impurities. In this article we will discuss the HPLC analysis of ONs using the Thermo Scientific™ DNAPac™ RP using LC-UV; LC-MS analysis will be discussed in Article 4 of this series.
The Thermo Scientific™ DNAPac™ RP column is a reversed-phase column designed for the high resolution separation of oligonucleotide and their failure sequences. These include oligonucleotide with modifications designed to improve stability in biological fluids, such as ribose modification, phosphorothioate (PS) substitution and linkage isomers. This column also supports gel-like separation of dsDNA fragments generated by restriction enzymes for cloning and next generation or high throughput sequencing (NGS, HTS). The column is based on a hydrophobic, polymer resin, so it is stable under high pH and/or high temperature conditions which often provide higher resolution for challenging oligonucleotide samples. In addition, the particles harbor a wide pore size (1000~2000 Å) to improve separation of large double-stranded nucleic acids used for cloning and NGS.
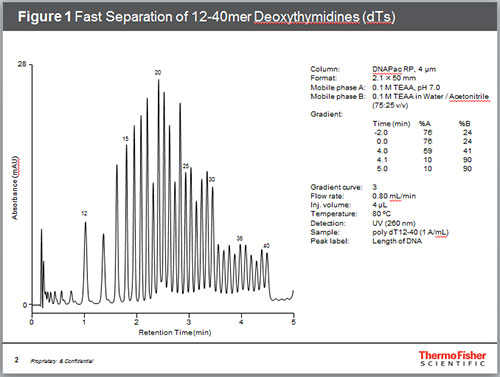
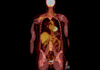
Figure 1. Fast Separation of 12-40mer Deoxythymidines (dTs)
Fast, High Resolution Separation of Single Stranded DNA
It is generally accepted that the ion-pair reagent such as triethylamine interacts with the stationary phase via hydrophobic interaction which in turn acts as an ion-exchange site for the negatively charged oligonucleotides. At pH values between 6 and 8, standard oligonucleotides have one negative charge for each phosphodiester bond. Therefore, a terminally non-phosphorylated oligonucleotide will harbor a charge equal to one less than the number of bases. Since the number of phosphodiesters, and thus the number of IP reagent molecule interactions, is proportional to the length of the oligonucleotide, longer oligonucleotides elute later at neutral pH values. As the oligonucleotides get longer, the percent charge difference between oligonucleotides decreases, so resolution between oligonucleotides decreases with oligonucleotide length. The separation of single stranded poly-deoxythymidine (dT) using IP-RP is illustrated in Figure 1 and Figure 2. A poly-dT 12-40mer was separated at high temperature and high flow rate with a 4-minute gradient using triethylammonium acetate (TEAA) as mobile phase. In addition, up to 60mer poly-dT oligonucleotide was separated using a 7-minute gradient. In both cases, a convex gradient was used to maximize the resolution of the longer oligonucleotides in shorter time.
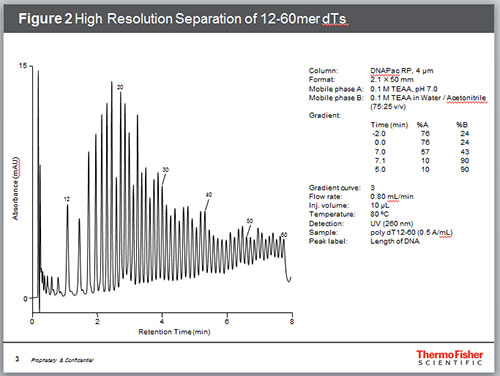
Figure 2. High Resolution Separation of 12-60mer dTs
Separation of Failure Sequences
Among the most common impurities in synthetic oligonucleotides are failure sequences. While each coupling step is highly efficient, the probability of coupling failures increases with each such step. Hence, one or two nucleotide “deletions” can be expected in most synthetic oligonucleotides. Figure 3 shows the separation of a 25mer DNA from its 5’ deletion sequences (n-1 and n-2).
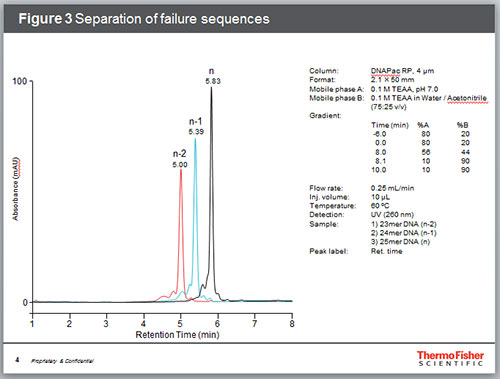
Figure 3. Separation of failure sequences
Separation of Fluorescent Dye-Labeled DNA
Fluorescent dye-labeled oligonucleotides are used in many applications such as DNA sequencing, PCR, DNA microarrays and in situ hybridization. In most cases, attachment of a fluorophore increases the overall hydrophobicity of the oligonucleotide sample which results in a stronger retention on a reversed-phase stationary phase. Figure 4 shows the separation of a fluorescein labeled oligonucleotide and its unmodified form. The unmodified oligonucleotide and other impurities were separated from the main dye-labeled oligonucleotide peak.
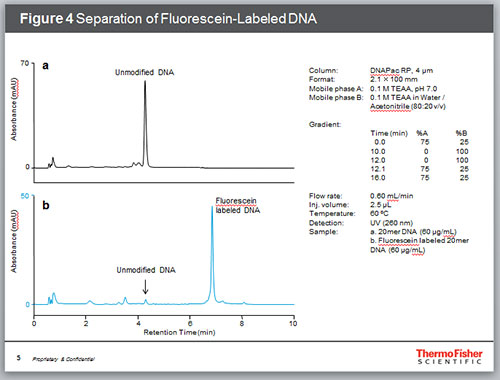
Figure 4. Separation of Fluorescein-Labeled DNA
Separation of Large Double-Stranded DNA Fragments
Purification and sizing of large double-stranded DNA fragments is an important step in DNA cloning, PCR and for preparation of DNA libraries for HTS or NGS. Traditionally, these dsDNA fragments are separated by agarose or acrylamide gels, and extracted from the gels for subsequent use. This process requires manual excision of the target DNA from the gel, and extraction of the DNA from the excised gel. This laborious and time consuming step generally produces yields of less than 50%. The DNAPac RP provides reliable separation and higher yields and DNA fragment collection is readily automated. Many reversed-phase columns advertised for such separations harbor small pores which restrict separation of dsDNA fragments over 600-1000 bp. The wide-pore DNAPac RP resin produces resolution comparable to many DNA gels up to 10K base pairs, and in similar times. Figure 5 shows the separation of dsDNA fragments generated from a restriction enzyme (top panel) and a DNA ladder (bottom panel). Fragments ranging from 72 bp to 10,000 base pairs were separated on the DNAPac RP in 15 minutes.
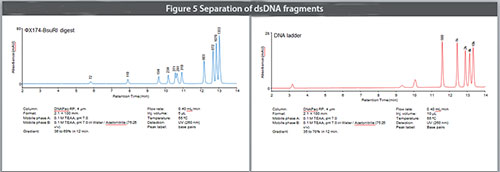
Figure 5. Separation of dsDNA fragments
Additional Resources:
A. DNAPac RP Product Specifications
B. DNAPac Family of Columns Brochure
Julia Baek, Ph.D. ([email protected]), is a research scientist, Jim Thayer, Ph.D. ([email protected]), is staff research biochemist, and Ilze Birznieks ([email protected]) is the global product manager of the Bio LC columns at Thermo Fisher Scientific.