February 1, 2010 (Vol. 30, No. 3)
pH Measurement System Features Intelligent Sensor Management & Digital Signal Transmission
The starting material for virtually all pharmaceuticals and biologics is a synthetic or chemical. Human plasma-derived therapies are one of the few exceptions. A number of fragile proteins can be extracted from human plasma. Indeed, no other single starting material can be utilized for producing such a diverse range of therapies as is possible from human plasma.
The albumin, clotting factors, immunoglobulins, and alpha-1 proteinase inhibitors that are fractionated from whole plasma are used to treat a wide spectrum of genetic, chronic, and rare diseases and conditions such as hemophilia, primary immunodeficiencies, burns, and shock.
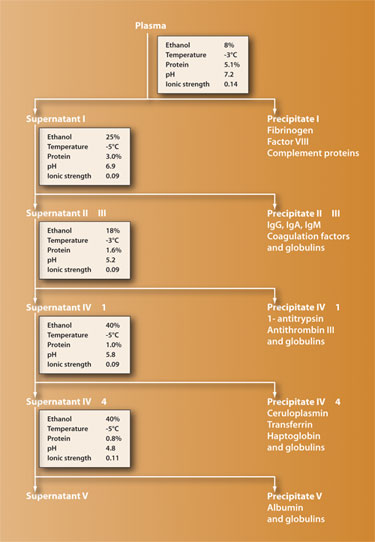
In order to produce plasma-derived therapies, plasma is typically first pooled from thousands of donors, purified using the fractionation process, and then treated by heat or with chemicals to inactivate viruses such as HIV and hepatitis. Fractionation of the plasma involves exploiting natural properties of the various proteins in such a way that they become insoluble in the plasma fluid. This method yields five major fractions that can be extracted and processed to inactivate blood-borne agents and produce specific products.
The first large-scale method for fractionation using ethanol was developed by Edward J. Cohn and his team during World War II, their goal being to extract albumin to be used as a replacement for whole plasma given to wounded soldiers.
The success of the process and efficacy of the albumin and other fractions led to many variations of the Cohn process being developed to elicit a greater quantity of purer proteins (a typical basic fractionation scheme is shown in Figure 1). Although common manufacturing steps exist across fractionators, there are no standardized processes for manufacturing the therapies, and each fractionator has adopted its own methodology in order to maximize the yield, purity, safety, and efficacy of its products.
Whatever the recipe, plasma fractionation is a complex process that is unique to plasma-derived therapies. To precipitate the various proteins, ethanol concentration, protein concentration, temperature, pH, and ionic strength are all modified at various stages. Despite its age, obtaining therapies by cold ethanol plasma fractionation is today by far the largest segment in the global therapeutic protein industry. Gel filtration, ion-exchange chromatography, and affinity absorption are more precise protein-purification methods, but these are laboratory procedures that do not easily scale up to meet the financial requirements of pharmaceutical companies.
Variations on five parameters are used to regulate the plasma-alcohol pool in order to affect the solubility of the proteins:
- ethanol concentration ranging from 8% to 40%
- temperature from -3° to -5°C
- protein concentration from 5.1% to 0.8%
- pH level from 4.8 to 7.2
- ionic strength ranging from 0.14 to 0.09
Processing is usually by batchwise treatment of the solution in stirred tanks (although a continuous flow fractionation method exists, most producers have abandoned it) and once all the fractions have been precipitated, the ethanol is removed and reused.
As mentioned previously, pH is one of the critical parameters for efficient fractionation. Although pH can be monitored using grab samples, this is a time-consuming method for technicians and can also lead to a delay in the detection of an incorrect pH level, which could ultimately affect yield, quality, or both. Off-line sampling also carries a risk of contamination.
Therefore, the benefits of continuous real-time measurement provided by an in-line pH measurement system are obvious. However, using in-line measuring equipment is not without its risks. Should the electrode fail during a batch, production has to be stopped while a replacement is calibrated and installed.
Mettler Toledo has developed a pH measurement system that not only eliminates the risk of electrode failure during production but also meets all stringent pharmaceutical requirements.
The newest generation of Mettler Toledo pH electrodes features intelligent sensor management (ISM) technology. A microchip within the head of the electrode continuously monitors the status of the sensor. The dynamic lifetime indicator display on the connected M400 transmitter clearly shows if an electrode can be safely used for the next batch.
ISM electrodes can be precalibrated outside the clean zone and stored for future use. Should an electrode need replacing, it is then a simple and quick task to exchange it. The plug-and-measure functionality ensures that the new electrode can be used for measuring as soon as it is placed in the plasma solution, thus also helping to ensure production uptime is maximized.
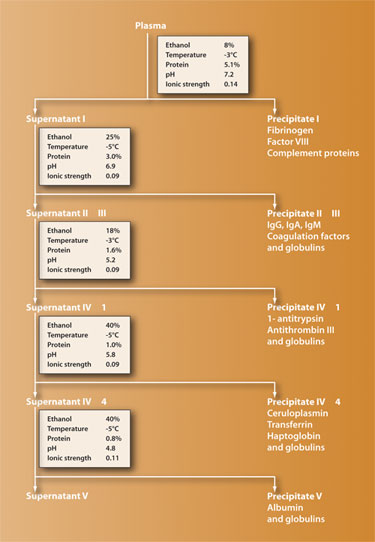
Figure 1. Basic plasma fractionation scheme using the Cohn process
The microchip in ISM-equipped electrodes includes an analog-to-digital converter. Low-impedance, digital transmission of the sensor signal is reliable, even in humid environments. Further, the memory built into the electrode allows the storing of calibration information. A PC-based database stores data on all electrodes such as the number of cleaning cycles or total operating time, allowing full traceability.
The electrical resistance of pH-sensitive glass is highly temperature dependent (as shown in Figure 2). A special low-temperature glass, such as that used on the InPro 3250i electrode (Figure 3), is needed for the conditions present during protein precipitation to ensure an acceptable resistance and accurate measurement regardless of temperature, or ethanol or protein concentration.
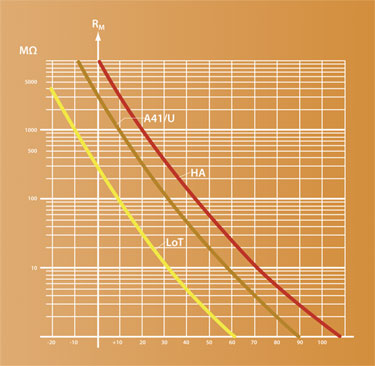
Figure 2. Relationship between electrical resistance and temperature for three types of pH-sensitive glass
As the electrode is in continuous contact with the medium, hygienic design is a vital requirement to prevent contamination during production. Special attention has to be made to the housing regarding surface quality, O-rings, and materials. Fractionation runs in stainless steel vessels where side-entry mounting is possible. The European Hygienic Engineering and Design Group develops test methods that are used by Mettler Toledo for hygienic design assessment to aid compliance with relevant legislation.
pH electrodes with ISM technology, equipped with a low-temperature glass, low-temperature reference electrolyte, and digital (low-impedance) signal, ensure reliable process control and are tailored for blood plasma fractionation. A modern transmitter interprets the digital signal from the pH electrode and delivers a defined signal to the process control system.
Blood plasma fractionation after Cohn and pH measurement with glass electrodes share a significant commonality—both technologies are more than 60 years old. In the decades that have passed, significant developments have been made to both. Blood plasma fractionation methods are continuously improving and new technologies such as chromatography have been introduced. Likewise, today’s pH measurement systems cannot be compared with those available in the 1950s. Electrodes with intelligent sensor management and digital signal transmission allow reliable and safe control, and improvements in materials ensure measurement accuracy and repeatability across a wide range of conditions.
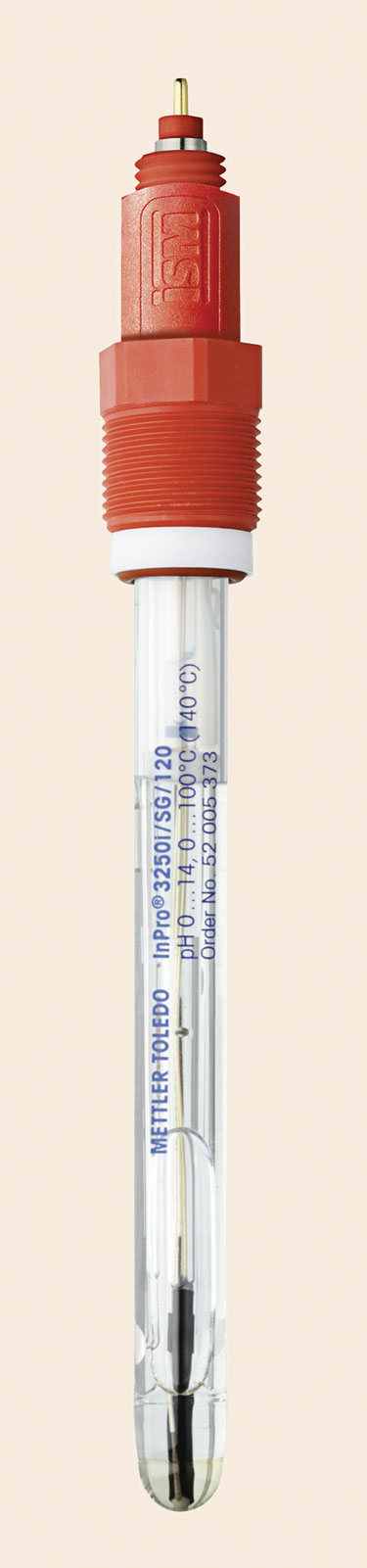
Figure 3. InPro 3250i pH electrode
Kurt Hiltbrunner ([email protected]) is market specialist pharmaceutical industry at Mettler Toledo. Web: www.mt.com/pro.