March 15, 2009 (Vol. 29, No. 6)
A DESI-MS Workflow Has Applications in Drug Candidate Qualification
Mass spectrometry (MS) is already perhaps the most popular analytical chemistry tool in drug discovery and development. Its use is ubiquitous in bioanalytical laboratories for drug and metabolite quantitation, and it has enabled rapid advances in global protein profiling and disease biomarker discovery. The implementation of electrospray ionization (ESI) and matrix-assisted laser desorption/ionization (MALDI) have been catalysts for the rapid expansion of MS-based applications.
ESI enables solution-phase analytes to be introduced as ions into the mass spectrometer for mass analysis, while MALDI creates ions of the analytes in the sample under vacuum conditions by sublimation of a crystalline matrix using laser pulses. Developments in the MALDI method over the last ten years have resulted in the ability to image intact tissue sections for proteins and peptides.
The analysis of tissue samples by MALDI for small molecules is particularly challenging given the high chemical background that results from having to prepare the tissue section with a suitable matrix for proper extraction and crystallization of the analyte. The time, skill, and equipment required for proper sample preparation can also be cumbersome. As a result, new methods for direct sample analysis have been developed that aim to circumvent some of these challenges.
Until very recently it was considered impossible to sample and image surfaces with mass spectrometry at ambient pressures and temperatures. This tutorial will explore the principles of desorption electrospray ionization (DESI) mass spectrometry, which has been used to scan across the surface of a tablet, image an untreated tissue section with 230 µm lateral resolution, and was even featured on the television series CSI:Miami to image a latent fingerprint.
Desorption Electrospray Ionization
The basic conceptual arrangement of the DESI method is shown in Figure 1a. In DESI, a spray nozzle is used to direct charged droplets formed by an electrospray in the laboratory atmosphere toward the sample surface. The impact and interaction of the charged droplets at the surface causes analytes to be removed in smaller progeny droplets that are then swept into the inlet of the MS for mass analysis.
The Omni Spray® 2D Ion Source from Prosolia incorporates the DESI method (Figure 1b). The Omni Spray 2D Ion Source is automated in the X- and Y- axis and has the capacity to sample surfaces having up to a 114 mm x 76 mm footprint. The surface platform can accept microtiter format sample plates, such as the Omni Slide™-96, or up to three standard microscope glass slides.
The Omni Spray 2D Ion source is software controlled with an intuitive graphical user interface (GUI). Figure 1c shows the GUI in a multisample scanning mode typically utilized in tissue-imaging experiments. This feature allows multiple samples of various dimensions each with its own user-configurable motion control parameters (e.g., scanning velocity) to be set-up in sequence. In a typical imaging experiment, the surface is moved in one direction beneath the DESI ion source, while recording mass spectral data on a row-by-row basis.
Prosolia’s FireFly™ data-conversion software (Figure 1d) is an essential tool for converting raw mass spectral data recorded using the Omni Spray 2D Ion Source into a useful format for image visualization and manipulation.
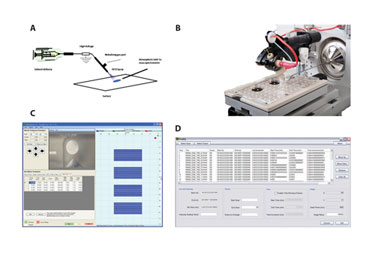
Figure 1: A–D
Experiment
Male Sprague-Dalley rats were given 50 mg/kg clozapine PO and sacrificed 30 minutes post-dose. The brain was extracted, immediately frozen over liquid nitrogen, and cut into 10 µm slices using a cryo-microtome. Each tissue section was thaw-mounted onto a plain microscope glass slide and analyzed without further processing.
Tissue-imaging experiments were conducted using a prototype version of an automated, two-dimensional DESI ion source like the one depicted in Figure 1b. The data was acquired using a Thermo-Fisher Scientific LTQ linear ion trap mass spectrometer using positive ion detection. The DESI ion source parameters were as follows: tip to surface distance: 2 mm; incident angle: 55°; solvent flow rate: 2 µL/min; and and nebulizing gas back pressure: 100 psi (7 bar). Raw data files acquired in Xcalibur™ were processed using FireFly (Figure 1d).
Drug Imaging
In the drug candidate qualification process, pharmacokinetic screening, metabolic stability with metabolite identification, enzyme induction and inhibition, and excretion studies play a major role. For tissue-distribution studies, whole-body autoradiography (WBA) is commonly employed. It has the advantage of being quantitative in nature but suffers from several disadvantages.
In particular, WBA requires the use of radioactive compounds and turnaround times can be up to several weeks. DESI-MS imaging presents an alternative approach for tissue-distribution studies by allowing for direct analysis and imaging of intact tissue sections without the use of radio-active labels. Sample turnaround times of just a few hours are another benefit. A typical mass spectrum recorded on a 10 µm thick rat-lung tissue section is presented in Figure 2a.
The lower m/z range represents the region where most drugs and metabolites are detected. The upper portion of the m/z range represents the lipid and peptide region. Figure 2b shows the DESI mass spectrum in the lower m/z range of the lung tissue. Of particular interest are the peaks at m/z 327 and m/z 313, which represent the presence of the drug clozapine and its metabolite, desmethylclozapine, respectively. Among other possibilities, the remaining peaks include endogenous small molecules carrying a positive charge.
Figure 3 shows the optical image and the DESI image from a rat brain section taken from an animal that had been dosed at 50 mg/kg of clozapine via oral gavage and the brain removed 30 minutes post-dose. The tissue was imaged in the MS/MS mode, and the DESI product ion spectra resulting from fragmentation of clozapine at m/z 327.1 (M+H)+ were recorded with a pixel size of 245 µm x 245 µm.
The Omni Spray 2D Ion Source has advantages and presents possibilities for the drug discovery scientists’ toolbox. Utilizing the Omni Spray 2D Ion Source for tissue-distribution studies has the potential to eliminate the need for expensive synthetic molecules while increasing sample throughput. DESI has also been used for exploring the impact of drugs and disease on endogenous compounds, revealing counterfeit drug formulations, and identifying thin layer chromatography spots. In addition, the technology has shown potential in pharmacokinetic screening and cleaning validation.
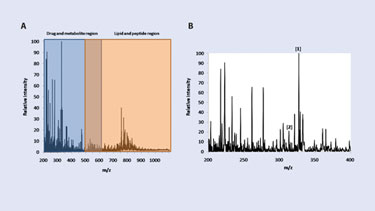
Figure 2. A–B
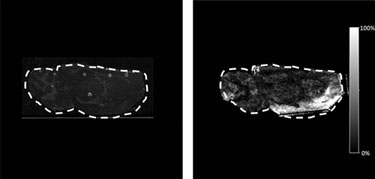
Figure 3. A and B
Justin M. Wiseman ([email protected]), is director of R&D, and Peter T. Kissinger is chairman/CEO at Prosolia. Web: www.prosolia.com.



