December 1, 2008 (Vol. 28, No. 21)
Andrew L. Niles Senior Research Scientist Promega
Simon M. T.M. Allard Ph.D.
Jason Greene
Sarah Shultz
New Tool Assesses Cell Viability Cytoxicity, and Apoptosis
Cell-based assays are useful tools to investigate in vitro responses to a variety of target compounds and cell-signaling molecules. Multiparametric measures are often desirable, particularly in secondary screening, to generate more biologically relevant data. The evaluation of multiple parameters can become quite labor intensive and time consuming if conducted in parallel, so the use of simple multiplex assays executed with automation help to improve efficiency and reduce operator involvement.
In this tutorial, we introduce an automated triplex cell-based assay application for simultaneously measuring three parameters: cell viability, cytotoxicity, and apoptosis. The method combines two fluorescent and luminescent assay chemistries—Caspase-Glo® 3/7 and MultiTox-Fluor™ Assays—offered by Promega, in the same assay well to extract information about viability, cytotoxicity, and caspase activation events. These parameters are particularly useful to define mechanisms associated with a cytotoxic profile. All dispensing steps are automated and performed using the Precision™ XS Automated Sample Processor (BioTek).
Triplex Assay
The triplex assay described in this article is comprised of the Promega MultiTox-Fluor and Caspase-Glo 3/7 Assays (Figure 1). The MultiTox-Fluor Assay is a nonlytic chemistry that allows users to measure live and dead cells in a single sample well. Specifically, for live cell assessment, live-cell protease activity is measured by the fluorogenic, cell-permeant peptide substrate Gly-Phe-7-amino-4-trifluoromethyl coumarin (GF-AFC). This live-cell protease activity marker labels only live cells because it becomes inactive upon loss of membrane integrity and leakage into the surrounding culture medium, and thus does not contribute to the dead-cell measurement. For dead-cell assessment, a second protease activity marker, the cell-impermeant peptide substrate bis-(Ala-Ala-Phe)-rhodamine 110 (bis-AAF-R110), is used to measure the activity of a dead-cell protease from cells that have lost membrane integrity and leaked the biomarker into the surrounding culture medium. Following use of the MultiTox-Fluor Assay, a second assay is performed to obtain a third type of data from the cell population. The Caspase-Glo 3/7 Assay is a luminescent assay that measures caspase-3 and -7 activities in cultures of cells, which are indicative of apoptosis. The assay provides a proluminescent caspase-3/7 substrate, which contains the tetrapeptide sequence DEVD. This substrate is cleaved to release aminoluciferin, a substrate of luciferase used in the production of light. The amount of light produced correlates with caspase-3/7 activity. Together, these assays provide a researcher with three pieces of data per well (cell viability, cytotoxicity, and caspase activity), which can be used to more accurately profile compound affects on cells. The triplex assay also has a built-in control for assay normalization to help correct for well-to-well and day-to-day variability within a cell-based system.
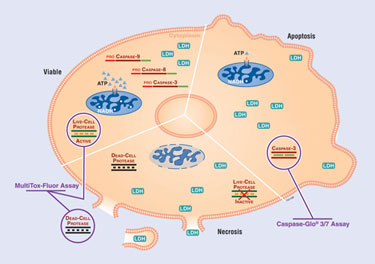
Figure 1. The triplex assay principle using the MultiTox-Fluor and Caspase-Glo 3/7 Assays.
Automated Dispensing Instrumentation
The BioTek Precision XS Automated Sample Processor (Figure 2) is a precise liquid-handling system used for dispensing steps. It is easily programmed to implement the triplex assay. This compact robotic instrument combines a single-channel sample processing head, an eight-channel pipetting head, and an eight-channel bulk reagent dispenser to control all liquid-handling steps of the assay including the dilution of compounds, sterile dispensing and treatment of cells, and addition of assay reagents. The small footprint of the Precision XS enables sterile experimentation inside a biological safety cabinet, which is critical for any assay with treatment times beyond 12–24 hours. The Precision Power™ PC software that accompanies the Precision XS Automated Sample Processor makes stepping through the assay protocol straightforward and easy. Specifically, a five-step protocol was created on the Precision XS instrument for these triplex experiments. All dispensing steps were performed using the BioTek Precision XS instrument.

Figure 2. BioTek Instruments’ Precision XS Automated Sample Processor
Compounds and Treatment Times
The effects of two compounds (ionomycin and staurosporine) over four treatment time points (4, 8, 24, and 48 hours) were selected for this triplex assay.
Ionomycin was selected because it causes primary necrosis in less than five hours, typically with no caspase activation. Ionomycin is a bacterial ionophore commonly used in research to affect calcium transport across cell membranes. Ionomycin also stimulates production of inflammatory cytokines. Ionomycin is a commonly used cytotoxic agent. Staurosporine is an antibiotic and pleotropic ATP-competitive kinase inhibitor that causes caspase activation at five to eight hours by the inhibition of protein kinases through the prevention of ATP binding. Like ionomycin, staurosporine is considered a standard cytotoxic agent.
Along with compound concentration, timing is an important factor to include in a comprehensive evaluation of the effects of a drug on a population of cells. Multiple time points enable researchers to more carefully observe reduction in replicative rate as well as changes in caspase activity without appreciable cell death. Because the mechanism of cell death is unique for each compound, the triplex assay presented here allows researchers to evaluate the mechanism by assessing viability, cytotoxicity, and caspase activity at each time point to understand what is happening inside the cell.
For example, apoptosis (typically 4–48 hours) can cause a time-dependent reduction in the activity of cell-viability markers often with a proportionate increase in cytotoxicity marker activity, as a result of caspase activation. Necrosis presents a similar profile, but in a much shorter time frame (i.e., < 4 hours) without caspase activation. Thus, being able to examine cellular cytotoxicity response kinetics using same-well multiplexed assays can bring new, more comprehensive insight to compound profiling and cellular responses.
Protocol
K562 cells were plated at a density of 10,000 cells per well in 50 µL volumes of RPMI1640 plus 10% fetal bovine serum and allowed to attach for two hours. Saurosporine and ionomycin were twofold serially diluted and added to wells in 50 µL volumes. Plates were incubated at 37°C in 5% CO2 for 4–48 hours. MultiTox-Fluor™ Reagent was prepared as 10 µL of each substrate in 1 mL assay buffer, and 10 µL was used per well. The plate was mixed and incubated for 30 minutes at 37°C. Fluorescence as measures of viability and cytotoxicity was read at two wavelengths on a Tecan Safire2 reader. Caspase-Glo 3/7 Reagent was then added in an additional 100 µL volume, and luminescence was measured after 30 minutes at room temperature on a GloMax®-Multi Plate Reader.
1. Sterilely dispense 50 µL K562 cells in RPMI1640 plus 10% FBS culture medium into clear-bottom white tissue culture treated 96-well assay plates at a density of 10,000 cells per well.
2. Manually dispense 200 µL test compounds at double the highest desired concentration into column 12 wells of a second 96-well plate. Backfill (100 µL medium plus carrier) and generate serial dilutions of compounds across the plate by transferring 100 µL to the neighboring well, mixing, and repeating. Transfer 50 µL prepared compounds to respective wells of the assay plate containing cells. Shake the plate briefly on an orbital shaker and place at 37°C and incubate for the desired test exposure period (4, 8, 24, or 48 hours).
3. After the desired treatment time, dispense 10 µL MultiTox-Fluor Reagent to all wells, shake briefly, incubate at 37°C for 30 minutes, and measure resulting fluorescence using a fluorometer (live-cell fluorescence 400Ex/505Em; dead-cell fluorescence 485Ex/520Em). The MultiTox-Fluor™ Reagent was prepared by adding 10 µL of each substrate to 1 mL of assay buffer for this triplex protocol, see Promega Technical Bulletin #TB348 for details.
4. Dispense 100 µL Caspase-Glo® 3/7 Reagent to each well of the assay plate, orbitally shake briefly to lyse the cells, incubate at room temperature for 30 minutes, and measure luminescence using the GloMax®-Multi luminometer.
5. Repeat Steps 3 and 4 after each respective treatment time.
Results
The triplex assay described in this article is comprised of the Promega MultiTox-Fluor and Caspase-Glo 3/7 Assays (Figure 1). The MultiTox-Fluor Assay is a nonlytic chemistry that allows users to measure live and dead cells in a single sample well. Specifically, for live cell assessment, live-cell protease activity is measured by the fluorogenic, cell-permeant peptide substrate Gly-Phe-7-amino-4-trifluoromethyl coumarin (GF-AFC). This live-cell protease activity marker labels only live cells because it becomes inactive upon loss of membrane integrity and leakage into the surrounding culture medium, and thus does not contribute to the dead-cell measurement. For dead-cell assessment, a second protease activity marker, the cell-impermeant peptide substrate bis-(Ala-Ala-Phe)-rhodamine 110 (bis-AAF-R110), is used to measure the activity of a dead-cell protease from cells that have lost membrane integrity and leaked the biomarker into the surrounding culture medium. Following use of the MultiTox-Fluor Assay, a second assay is performed to obtain a third type of data from the cell population. The Caspase-Glo 3/7 Assay is a luminescent assay that measures caspase-3 and -7 activities in cultures of cells, which are indicative of apoptosis. The assay provides a proluminescent caspase-3/7 substrate, which contains the tetrapeptide sequence DEVD. This substrate is cleaved to release aminoluciferin, a substrate of luciferase used in the production of light. The amount of light produced correlates with caspase-3/7 activity. Together, these assays provide a researcher with three pieces of data per well (cell viability, cytotoxicity, and caspase activity), which can be used to more accurately profile compound affects on cells. The triplex assay also has a built-in control for assay normalization to help correct for well-to-well and day-to-day variability within a cell-based system.
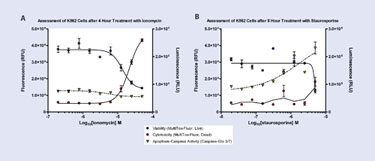
Figure 3A and 3B. Triplex assay results
Sarah Shultz ([email protected]) is a senior automation scientist in the integrated solutions and engineering department, Andrew Niles is a senior R&D scientist in the cellular analysis department, and Simon T.M. Allard, Ph.D., is a product associate in the cellular analysis & HTS/pharma marketing department, all at Promega.
Web: www.promega.com. Jason Greene is a product manager for liquid-handling and automation products at BioTek Instruments. Web: www.biotek.com.



