April 15, 2007 (Vol. 27, No. 8)
PET and SPECT Help Enhance the Quality of a Product and Prevent Late-stage Failure
Drug discovery and development is recognized as a long, costly, and risky process. Pharma is currently facing serious pipeline challenges characterized by rising R&D costs and declining productivity in new drug approvals. It now takes about 10–15 years and almost $1B to bring a new drug to market. Much of this expense is the result of costly late-stage failures. By 2010 the projected cost of successfully developing a new drug could reach $2B unless the efficiency and effectiveness of the drug development process are improved.
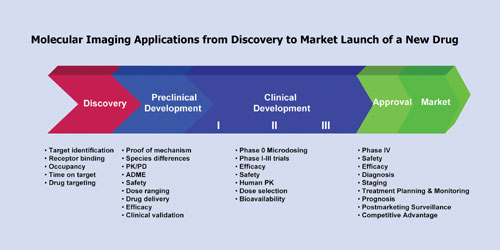
Over the past decade pharma has been introducing new technology tools (genomics, proteomics, metabolomics, biomarkers, and bioinformatics) into drug discovery and development, aiming to optimize the process and overcome the high costs of attrition. Among these tools, molecular imaging is maturing into an important enabling technology with expanding applications across the discovery and development continuum from validation of a druggable target through to market launch of a new drug (Figure 1).
Molecular imaging encompasses a variety of different modalities that can be categorized as follows:
• Anatomical modalities image structural morphology of organs or tissues, including computerized tomography (CT), magnetic resonance imaging (MRI), and ultrasound
• Functional modalities visualizing biological processes within organs or tissues at the cell and molecular level, including single photon emission tomography (SPECT), positron emission tomography (PET), and optical imaging (bioluminescence, fluorescence)
• Multimodality techniques combining structural and functional imaging capabilities enabling accurate localization of biological or pathophysiological processes in tissues or organs, including PET/CT, SPECT/CT, and MRI/PET.
Nuclear imaging techniques are currently the most advanced and widely used clinical imaging modalities. Recent introduction of complementary preclinical imaging equipment for small animals has resulted in PET and SPECT becoming pivotal enabling technologies for translational research and medicine particularly in oncology, neurology, and cardiovascular diseases.
Translation of preclinical results to clinical trials represents one of the most challenging stages in drug development, where failure rates from lack of safety or efficacy can reach 50%. PET and SPECT effectively bridge this transition to provide in vivo information on drug effects in experimental animals and humans using the same imaging modality and readout early in the development process. This not only enhances the quality of lead candidate selection but reduces late-stage failures by shifting attrition to earlier, less expensive stages of the development pathway. In the learn-and-confirm drug development paradigm, clinical data from Phase 0 microdosing or Phase I imaging studies can also be “back-translated” to optimize preclinical development.
Figure 1
Radiotracer Design
Nuclear imaging with PET or SPECT relies on use of radiolabeled tracers to visualize specific biological processes at the cell and molecular level. Radiotracer design and development are therefore key considerations. Generally three different approaches can be taken to develop radiolabeled tracers for use in drug development: 1) use the radiolabeled drug candidate to assess biodistribution and PK/ ADME characteristics; 2) use a radioligand for the drug target to assess PD properties of the drug candidate; 3) use a radiolabeled biomarker to assess efficacy of the drug candidate.
Isotopes used for imaging are either gamma emitters for SPECT imaging or positron emitters for PET imaging. A positron is a transitory beta-like nuclear particle that travels a few millimeters from its atomic nucleus source and is captured by an electron at which point the electron and positron are annihilated resulting in the generation of two photons that leave the point of capture in opposite directions.
When selecting radioisotope for nuclear imaging studies, several factors must be taken under consideration:
• the physical half-life of the radioisotope must be sufficiently long to meet the study requirements
• the biological half-life of the molecule to be radiolabelled
• the physical and chemical characteristics of the radioisotope such as type of radiation, isotope-specific activity, and trace metal contaminants that may be present with the radioisotope that can interfere with radiolabelling.
Some radioisotopes for molecular imaging are particularly short-lived (e.g., 11C 20 minutes and 18F 2 hours), which necessitates having the study carried out in close proximity to a cyclotron. Radioisotopes with half-lifes exceeding 12 hours can be shipped across continents from commercial suppliers to molecular imaging centers. Generator systems employ a longer-lived parent radioisotope that is stably retained in the system, from which a shorter-lived daughter radioisotope can be extracted. Such generator systems can be placed in imaging centers and conveniently provide radioisotopes on demand. Generators are particularly attractive for molecular imaging in areas where a cyclotron is not available. For SPECT imaging the 99Mo/99mTc generator is well-established and for PET the 68Ge/68Ga generator system is emerging.
Irrespective of the study objective it is desirable to engineer a true tracer, one that replicates the drug or biomarker’s nature. The radiolabel must not influence the biological or pharmacological activity of the drug candidate or biomarker. In this regard 11C is an ideal radioisotope for imaging as it can be synthesized into a candidate drug for imaging. Unfortunately the short half-life of 11C restricts its use to molecules that can be synthesized relatively quickly and for imaging studies of short duration. Nevertheless 11C is the isotope of choice for small molecules and particularly for PET neurology imaging.
Radiohalogens (for PET imaging 18F, 76Br, or 124I; for SPECT imaging 123I) may be used. The most commonly used radio-halogen biomarker radiopharmaceutical for molecular imaging studies is 18F-fluorodeoxyglucose (FDG) that images hexokinase activity.
Frequently Iodogen or Chloramine T reactions are used to radiolabel the halogen onto the molecule, but unfortunately, for large biomolecules, such as antibodies, in vivo dehalogenation is a major issue. For biomolecules, such as antibodies, a preferred approach for PET imaging is to use 64Cu or 68Ga where the metal ion is stably held by a bifunctional chelate (BFC). BFCs serve two purposes. One part of the molecule covalently binds to the targeting molecule. The other part of the molecule binds the radioactive metal ion (Figure 2).
In selecting the appropriate BFC, consideration must be given to the targeting molecule, the radioisotope, and the effect on the target protein stability that conjugation and radiolabeling require. DOTA derivatives are pictured in Figure 3. In general, they are superior to acyclic diethylenetriaminetetraacetic acid (DTPA) derivatives which are also commonly used as BFCs. The choice of BFC is not trivial because some do indeed work better than others. Loss of radioactive metal ion is one of the main modes of degradation in vivo, which leads to inferior image quality. A review of the scientific literature is required to discern the best chelate for a given metal and targeting agent.
With regard to conjugation, most BFCs contain reactive groups that react with the e-NH2 group on lysine residues (A, B, and C in Figure 3). There is a trade-off between reactivity and stability with these compounds. SCN-Bn-DOTA is relatively stable in solution, even at pH 8. However, reactions with lysine residues are on the order of hours. DOTA-TFP-ester is intermediate with respect to reactivity and solution stability. DOTA-NHS-ester is so reactive that reactions with lysine residues are done in minutes. However, this BFC also reacts quickly with water, so inevitably degradation of the BFC occurs in solution.
There are other strategies to conjugate BFCs to targeting molecules. NH2-Bn-DOTA (D) can be coupled to the free carboxylic acid groups of aspartate or glutamate residues. DOTA-Maleimide (E) will react solely with the free sulfhydryl group in cysteine residues. Many protected DOTA derivatives are available (F) to conjugate to peptides using standard peptide synthetic methods.
Range-finding experiments must be performed to optimize the conjugation conditions. After purification of the conjugate it must be characterized. Range-finding experiments are then performed to determine the best conditions to radiolabel the conjugate, which include receptor binding studies. Methods to purify and characterize the radiolabeled drug product must be developed.
Nuclear imaging may be used in discovery, preclinical, and clinical development. Figure 4 demonstrates the use of dual-modality small animal SPECT/CT imaging for biodistribution studies in preclinical development. The application will dictate whether GLP or GMP guidelines must be followed. The quality of the final product is highly dependent on the quality of the starting materials. Preparing radiopharmaceuticals is no exception.
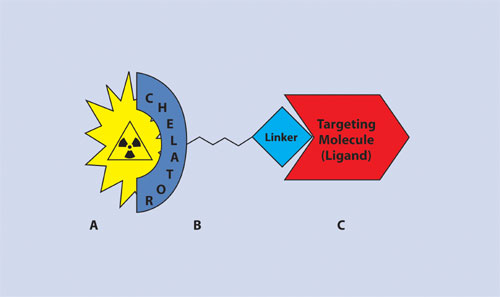
Figure 2
Conclusion
Nuclear imaging is proving to be a tool for drug development whose value is recognized by leading pharmaceutical and biotechnology companies and regulatory agencies. Ongoing advances in nuclear imaging are continually expanding the uses of PET and SPECT imaging in drug development.
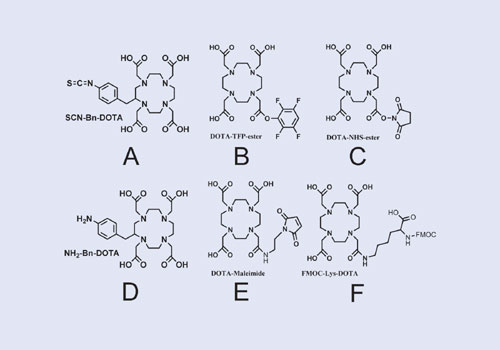
Figure 3
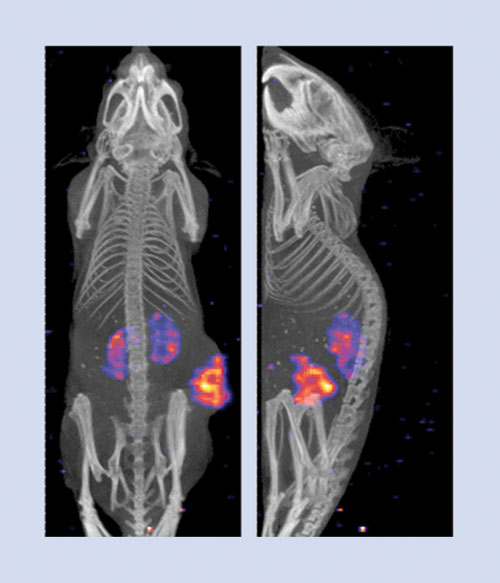
Figure 4
Lynn Buchanan, Ph.D., R&D at MDS Nordion, Paul Jurek, Ph.D., is quality manager at Macrocyclics (www.macro cyclics.com), and Russ Redshaw is director, business development at MDS Nordion. Web: www.mdsnordion.com. Phone: (613) 592-3400.
E-mail: [email protected].