December 1, 2016 (Vol. 36, No. 21)
To Understand What Is Happening During Treatment, Numerous Biomarkers Need to Be Analyzed Simultaneously
The activation of antitumor immunity by immune checkpoint blockade has demonstrated efficacy in a variety of cancers.
Although durable responses have been observed, combination approaches, to deepen or prolong responses, will be required to extend this benefit beyond a subset of patients.
In a typical combinational approach, the tumor is directly attacked while the immune system is stimulated. To understand biologically what is happening during treatment, numerous biomarkers need to be simultaneously analyzed.
Biomarkers are used to stratify patients, track target engagement in clinical trials, and guide decision-making and next steps. They provide pharmacodynamics as well as evidence in support of a proposed mechanism of action for a given treatment.
Tumors have evolved to evade the immune system; immunotherapies are designed to overcome this phenomenon. Developing biomarkers for cancer immunotherapy has unique challenges; the biocomplexity involves looking at both tumor and immune markers, and understanding intersystem relationships.
Biomarkers can offer clues about response to treatment. In some cases, prior to treatment, a tumor can be completely devoid of immune cells or have immune cells present only in the stroma. These tumor immune deserts generally do not respond well to immunotherapy. Monotherapy checkpoint inhibitors work best in more inflamed tumors. Inflamed tumors can have excluded immune cells, too.
A comprehensive biomarker plan for immunotherapy allows learning about these phenotypes and mechanisms of resistance that can potentially be targeted by entirely new approaches or through combinations with checkpoint inhibitors.
Common approaches involve looking at mutational load, PD-L1, and CD8+ T cells in the tumor and using gene expression to understand the phenotypes that are associated with some of the markers. Most recently, Genentech has begun exploratory work using multiplers, a flow-cytometry technique to follow antigen-specific T cells in the blood.
“We are starting to look at tumor antigen-specific T cells in the blood and combining this with other techniques such as T-cell receptor sequencing. Then we relate this information to what is going on in the tumor. It is more specific and brings you closer to the antitumor response. General blood markers are not as telling because they do not relate as well to efficacy in patients,” explained Jeffrey Wallin, Ph.D., group leader, cancer immunotherapy, early-stage oncology biomarker development.
“Biomarkers will continue to be a very important part of cancer immunotherapy, and will help us expand our understanding of biology and why some patients respond and other do not. The integration of the multiple marker approach will greatly increase knowledge; and combining markers, such as immunohistochemistry, with gene expression gives a spatial and functional context.”
Limitations and Approaches
Another challenge in development of predictive biomarkers for immuno-oncology (IO) are the limitations of the handful of mouse syngeneic models used in preclinical discovery. To overcome this limitation, OncoMed Pharmaceuticals has started to use their proprietary human xenograft tumor bank as an approach. The patient-derived xenograft (PDX) models are used in mice with a more humanized immune system, allowing immune effects to be studied.
“Once we identify what the biomarker is or have a strong hypothesis from the preclinical space, we take a modular approach to assay development. The key is to move as quickly as possible to get the assays in hand before you enter the clinic so you can start to test the biomarker with patient samples,” explained Ann Kapoun, Ph.D., vp, translational medicine.
“First, robust RUO assays are developed, and then the assays are moved into CLIA validation. This allows for clinical data collection and analysis before moving on to the more complex task of developing a companion diagnostic.”
OncoMed has an emerging pipeline in the IO space, including a T-cell activating GITR ligand. The company works to have a predictive biomarker in place for all of their programs. Depending on the biomarker strength it may be evaluated retrospectively in an exploratory fashion, prospectively during a clinical trial, or in a combination of the two approaches.
Biomarker development is still emerging in IO, as is how to use these biomarkers in combination therapy where the development of biomarkers is more challenging as the end game keeps changing, albeit also as an opportunity. No one size fits all; the mechanism of action and interactions of the drugs dictate needs.
IO is rapidly advancing, and tools and techniques need to keep pace. Better methods are needed to design predictive biomarkers for combination therapy as well as to track the resistance mechanisms that will certainly evolve.
Sequencing is another area ripe for refinement as methodology varies and standardization would greatly aid results comparisons.
No Biomarker Is Perfect
PD-L1 is the centerpiece of Merck’s IO biomarker program. The PD-L1 test, co-developed with Dako North America, is the first FDA-approved IO companion diagnostic, and enabled approval of Keytruda for non-small cell lung cancer. Merck is currently investigating PD-L1 as a predictive biomarker in more than 30 different tumor types.
(Keytruda had previously been approved for the treatment of patients with unresectable or metastatic melanoma and for patients with recurrent or metastatic head and neck squamous cell carcinoma).
“Specifically, we infer from the presence of PD-L1 that there was an initial cytotoxic immune response to the tumor, that tumor cells began to express PD-L1 as a counter-response to the initial immune response, that PD-L1 downregulated the immune response, and that Keytruda will reverse that downregulation, resuming the cytotoxic immune response,” said Kenneth Emancipator, M.D., executive medical director, companion diagnostics, Merck.
But no biomarker is perfect. Some patients whose tumors express PD-L1 do not benefit from Keytruda, and many patients benefit from Keytruda even though their tumors tested PD-L1 negative. Merck is investigating a number of other biomarkers that measure tumor inflammation or immunogenicity, including an interferon gamma signature, a mutational burden assay, POL gene mutations, and microsatellite instability.
The interferon gamma signature detects RNA from activated inflammatory cells, and measures the overall immune response to the tumor. The mutational burden assay is a count of the number of nonsynonymous somatic mutations and, presumably, the number of foreign antigens, which the tumor harbors. High mutational burden tumors are more likely to elicit an immune response.
POL gene mutations and microsatellite instability are markers for defects that cause high mutational burden. POL genes code for proofreading proteins, which detect errors in DNA replication. Microsatellite instability indicates defects in the proteins that repair those errors. The association between microsatellite instability and a familial form of colorectal cancer, Lynch syndrome, has been known for some time.
Within a few years, the question in cancer immunotherapy will shift toward whether the patient should receive anti-PD-1 monotherapy, anti-PD-1 combined with another immunotherapeutic agent, or anti-PD-1 combined with more traditional chemotherapy. Biomarkers will be crucial for choosing the therapy that provides the patient with the most benefit and the least amount of toxicity.
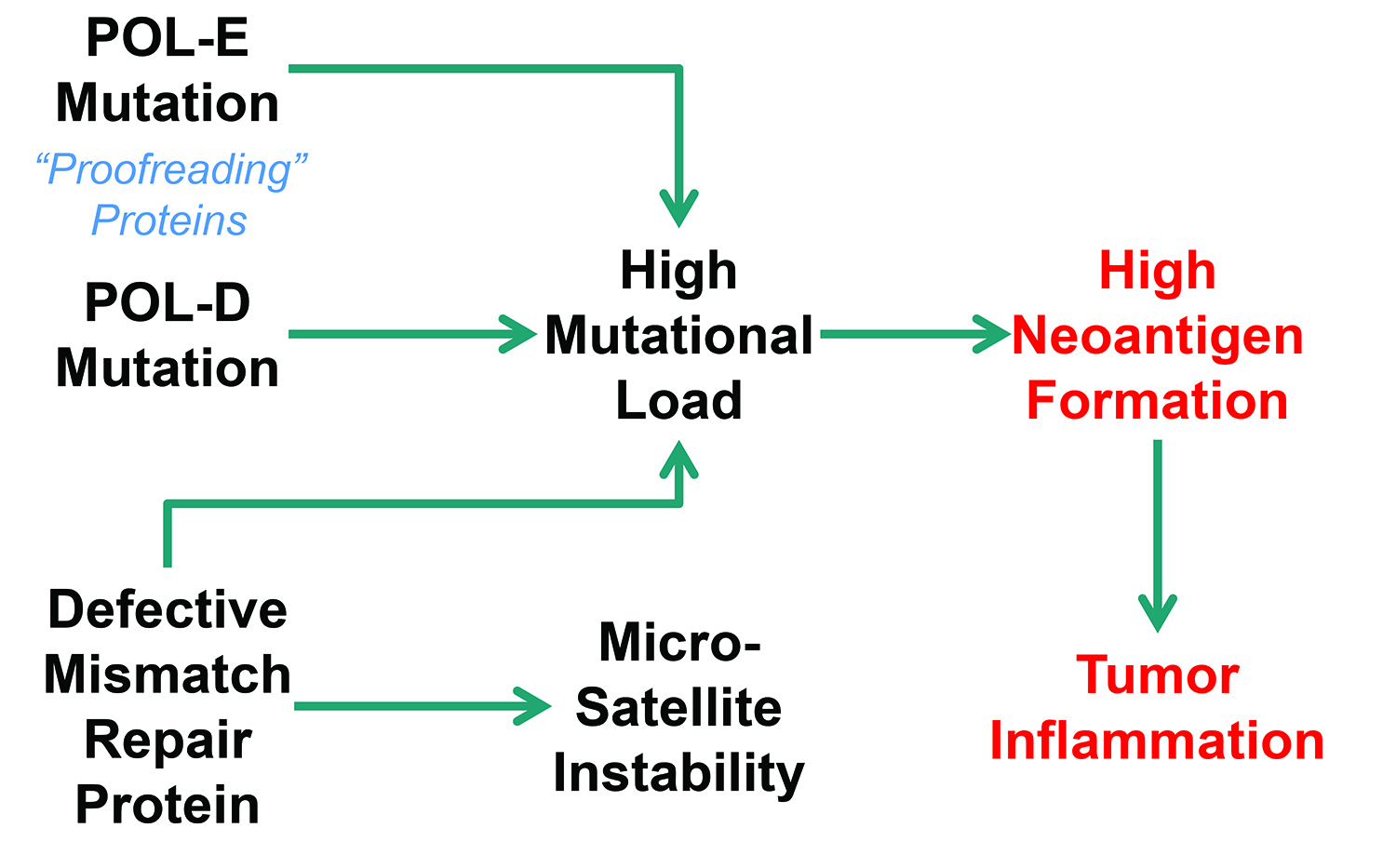
Merck is investigating POL gene mutations and microsatellite instability. POL genes code for proofreading proteins that detect errors in DNA replication, whereas microsatellite instability indicates defects in the proteins that repair those errors.
Faster Analysis
Combination immunotherapy will require monitoring of multiple biomarkers. While ELISAs are widely used, running multiple ELISAs in parallel requires large sample volumes and is not timely, practical, or cost-effective for analysis of multiple proteins.
ProteinSimple’s Simple Plex is a high-performance automated immunoassay platform consisting of disposable microfluidic cartridges that are run on the Ella instrument. The single-analyte cartridge measures a single biomarker in 72 individual samples, and the multi-analyte cartridge analyzes four biomarkers in parallel from 16 different samples.
The cartridges come pre-loaded with reagents and barcode-embedded, factory-calibrated standard curves. Reagent delivery for each step is precisely controlled using pneumatically actuated microfluidic pumps and valves.
The automated assays are performed within analyte-specific glass nano-reactors (GNRs). Each analyte ?
is measured by fluorescence quantitation of triplicate GNRs that are functionalized with capture antibody prior to being embedded in microfluidic channels.
In the multi-analyte cartridge, the sample is split into four independent microfluidic channels arranged in parallel; each channel has a dedicated well containing the analyte-specific detect antibody. This spatial separation eliminates potential cross-reactivity from antibody pairs, while the rapid microfluidic reaction kinetics ensure sensitive analyte detection from low sample volumes.
Time to results is a key criterion for monitoring IO biomarkers. Simple Plex reports concentration results in just over an hour, using 25 µL or less of sample. The specificity of the multi-analyte cartridge format provides accurate measurements of closely related protein family members.
“This is particularly relevant for IO, where panels of biomarkers may be used as signatures for particular immune cell types,” said Gregory Marusov, senior scientist, Simple Plex assay development, ProteinSimple. “For example, PD-L1 or PD-1 inhibitors are being widely explored to augment cytotoxic T-cell activation and antitumor immunity.
“In these cases, measurement of multiple cytokines and chemokines that together comprise a T-cell effector signature can be an important tool for understanding mode of action and for monitoring responses to immune checkpoint inhibition approaches.”
ProteinSimple has a broad menu of IO cytokine, chemokine, and other biomarker assays, and recently released a PD-L1 assay in response to customer demand.
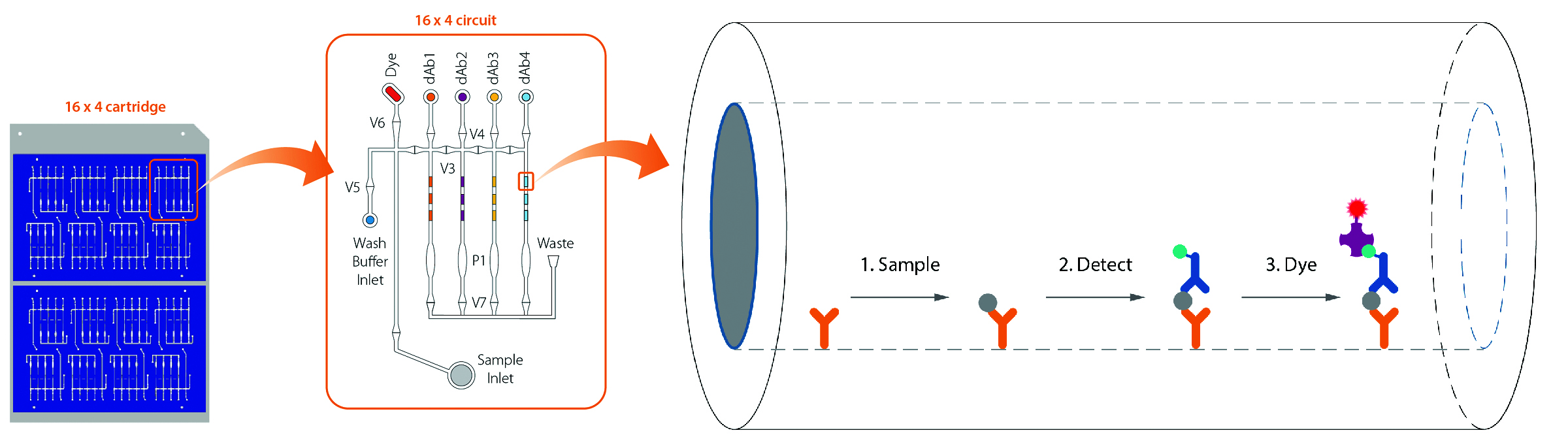
Simple Plex assays from Protein Simple are automated sandwich immunoassays that are performed within analyte-specific glass nanoreactors, or GNRs (right). A bottom view of the “16×4” multi-analyte Simple Plex cartridge is also shown (left). Each of the 16 samples is analyzed in an independent microfluidic circuit (middle), and reagent delivery within each circuit is precisely controlled in automated fashion by pneumatically actuated valves (V1–V7) and pistons (P1).
Accelerating Biomarker Discovery
According to the Cancer Research Institute, only six active immunotherapies have been approved for cancer, meaning that hundreds of other new and promising cancer immunotherapy treatments are only available to patients in clinical trials. Only 3–6% percent of cancer patients who are eligible for clinical trials participate. This slows the clinical development process significantly, and means that more than 90% of cancer patients may be missing out on potentially life-saving new treatments.
In the short term, casting a broad net to identify clinically relevant protein signatures can form the basis of more focused diagnostic/prognostic tests.
In some cases, it may be difficult to access material from the tumor; establishing less invasive, surrogate endpoint markers may be highly desirable. The protein panels from Olink Proteomics are designed for targeted human protein biomarker discovery and enable simultaneous analysis of 92 proteins, using only 1 µL of almost any type of biological sample.
The platform uses the Proximity Extension Assay (PEA) technology. Pairs of DNA-labeled antibody probes bind to their targeted protein, which brings the two probes in close proximity and the DNA labels will hybridize in a pair-wise manner. The addition of a DNA polymerase leads to a proximity-dependent DNA polymerization event, generating a unique PCR target sequence.
The resulting DNA barcode, representing a specific protein, is subsequently detected and quantified using a microfluidic real-time PCR instrument. This dual-recognition, DNA-coupled method provides exceptional readout specificity and enables the generation of high-quality data at a multiplexing level that is not feasible using traditional immunoassay techniques.
Each panel is focused on a specific area of disease or biology, targeting validated and exploratory biomarkers. Released panels include ones for inflammatory disease, cardiovascular disease, oncology, neurology, and, most recently IO. Panels are available as ready-to-use kits, or through Olink Proteomics’ in-house analysis service.
According to Ida Grundberg, Ph.D., business development manager, Olink Proteomics, the IO panel is the broadest protein panel available and can therefore provide a more complete picture.
Already used by researchers at Mount Sinai Medical School and Uppsala University, the panel provides assays that are relevant for key processes, such as promotion and inhibition of tumor immunity, chemotaxis, vascular and tissue remodeling, apoptosis and cell killing, metabolism, and autophagy, in addition to assays likely relevant for analysis of both plasma and the tumor microenvironment, explained Dr. Grundberg.
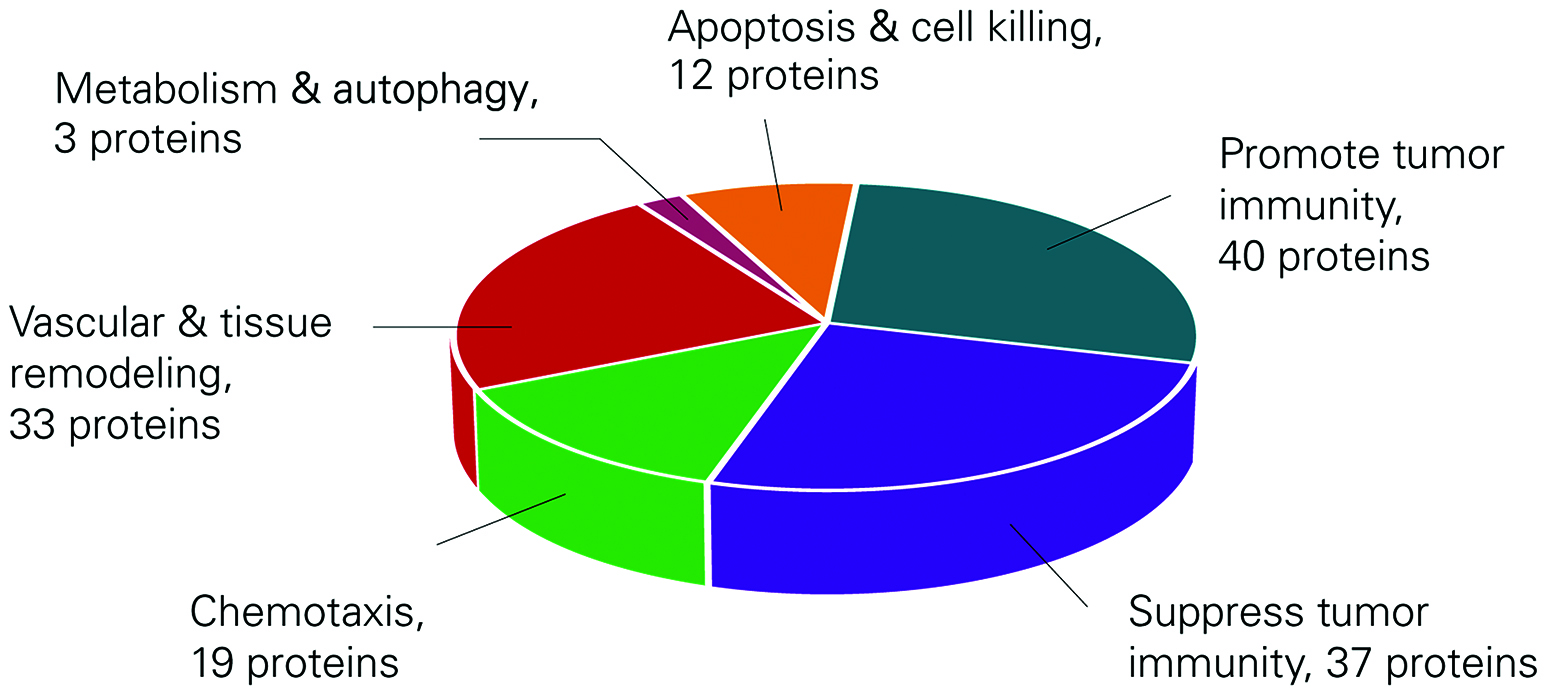
Olink Proteomics’ broad IO panel provides a detailed picture for biomarker discovery.
Scaling Up Viral Vector Production
Matt Kremer
Transduction of immune system cells to elicit a cellular immune response is a growing area of immunotherapeutic drug development. Most prominent are the autologous CAR-T (chimeric antigen receptor- T-cell) cellular therapies where a patient’s T-cells are isolated and transduced to express a tumor-associated antigen. When reintroduced into the patient, these cells elicit a powerful immune response against the target cancer cells.
Most CAR-T therapies use viral vectors to introduce the gene of interest into the target cell. Lentiviral and retroviral vectors are currently used for this purpose and are produced by transient transfection or in stable packaging cell lines.
Vectors are typically produced in adherent HEK cells using flatware for early clinical development. Scaling up from flatware for late clinical and commercial development is a challenge. One approach is to convert from adherent to suspension culture, which presents its own set of challenges; another is to take advantage of advances in large-scale adherent cell culture to scale up from the initial flatware process.
Scalable Platform
The iCELLis® fixed-bed bioreactor from Pall Life Sciences is a scalable platform for viral vector production in adherent cell culture. The iCELLis fixed-bed bioreactor is a closed and controlled single-use system supplied in two formats: the small-scale iCELLis Nano for process development and the iCELLis 500 for large-scale commercial production.
The fixed-bed is comprised of non-woven PET fabric strips. Depending on bed height and packing density, the Nano has surface areas of 0.53-4.0 sq/m; this corresponds to 66-500 sq/m in the 500 bioreactor, which measures 12 inches high by 36 inches in diameter.
Scaling from the Nano to the 500 is linear—similar to scaling a chromatography column—so that a process in the Nano can be directly transferred to the 500. Scaling from the iCELLis Nano to the iCELLis 500 has been demonstrated by FinVector, and adenovirus made in the iCELLis is now in clinical use in a Phase III clinical trial under an Investigational New Drug Application.
With specific regard to viral vectors for T-cell transduction, Memorial Sloan Kettering Cancer Center has reported results for retrovirus production in the iCELLis Nano system versus traditional flatware using stable packaging PG13 and HEK 293Vec cell lines.
Using iCELLis, the harvest window was extended to 10 days versus three days for flatware. Using 293Vec cells in a 2.7m2 Nano, the vector yield was 2.53×1012 versus 1.93×1011 in the flatware control. If scaled to the corresponding iCELLis 500 bioreactor with 333 m2 of available surface area, theoretical vector yield could reach 3.2×1016/batch.
For PG13 cells, reported vector production was 2.9×1010 in a 2.7m2 iCELLis versus 3.96×109 in flatware. For both cell lines, vectors produced in iCELLis passed the release test assays for clinical use.
In conclusion, the iCELLis system enables the scale-up of viral vector production from flatware with improved yields and consistent quality without major process changes such as switching to suspension culture. With more than 140 Nano systems in use worldwide as well as a growing base of iCELLis 500s, the publication of new techniques and applications can be expected.
Matt Kremer ([email protected]) is business development manager, cell culture technologies, Pall Life Sciences.
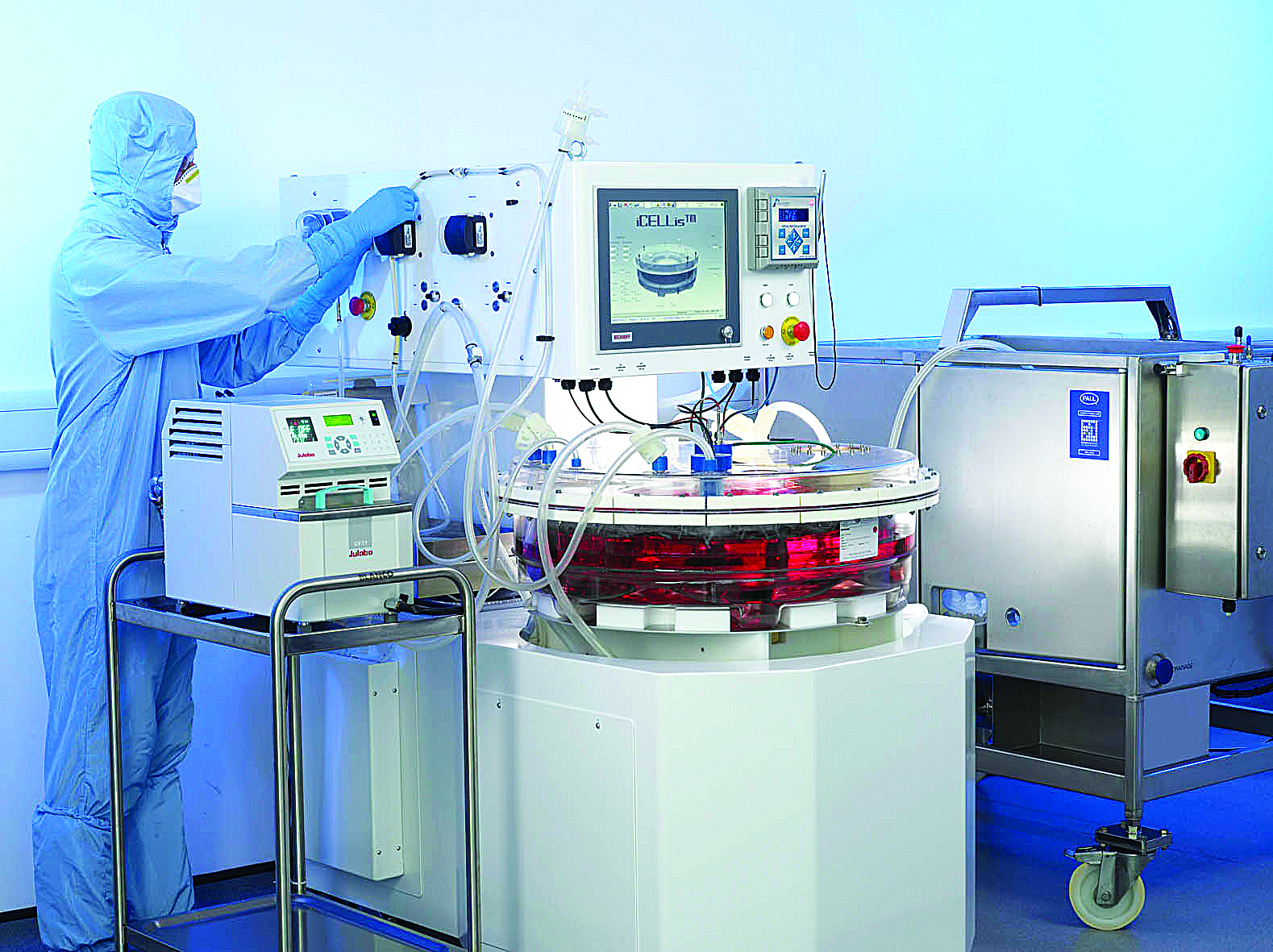
Memorial Sloan Kettering has used Pall’s iCELLis fixed-bed bioreactor for retrovirus production.



