May 1, 2014 (Vol. 34, No. 9)
Genetic modification aimed at understanding the function and pathology associated with specific genes is most commonly undertaken in mice, as about 99 percent of human genes have mouse orthologs.1 Traditionally, the most popular transgenic technique has been the constitutive knock-out, wherein the gene of interest is disrupted in the mouse. However, constitutive inactivation can deem mice unviable, thereby precluding the study of the effects of the gene. Researchers developed the conditional knock-out in response to this challenge, which allows a gene to be disrupted only in certain tissue or at a certain point in development.
Though knock-out and knock-in techniques in mice have provided powerful tools for genetic research, many studies cannot be properly translated to humans due to differences in the functions associated with mouse homologs of human genes. Modern transgenic technology has thus focused on genetic humanization, which allows the expression of human proteins in the mouse.
Humanization Techniques
The simplest way to generate humanized mice is to inject a transgenic construct harboring the human gene(s) of interest (e.g., part of a human bacterial artificial chromosome) into mouse oocytes. This allows the expression of human genes not normally present in the mouse (e.g., certain viral receptors, or target sequences for binding studies of human-specific biologics). However, this technique provides no control over where or how the gene integrates into the mouse genome, which can be problematic for a number of reasons.
The gene may land in the middle of an endogenous mouse gene, thereby inactivating it. Alternatively, the presence of the endogenous gene may cause interference and prevent faithful recapitulation of the gene function present in humans. To overcome this limitation, the technique can be combined with knock-outs,2,3 but an ideal approach would allow for direct homologous replacement. With such replacement, the human gene would replace the mouse ortholog at the same genetic location and produce a humanized allele.
Taconic has perfected this technically challenging approach of targeted gene replacement. Complete genomic replacement is particularly difficult to achieve if the gene of interest is large (i.e., greater than about 100 kilobases) because it is challenging to maintain the endogenous genomic set-up with larger genes. As it has been shown that retaining the exon-intron structure significantly enhances the likelihood of proper expression of the human protein, preserving such structure is an essential goal of gene replacement. Taconic can now offer single-step gene replacements in the 150 kb size range.
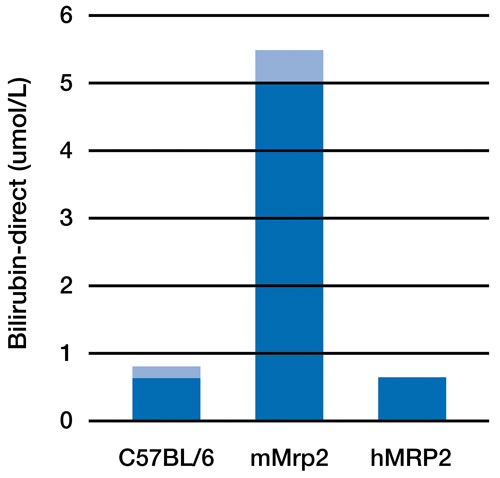
Figure 1. Conjugated bilirubin levels in mouse plasma significantly increase when the mMrp2 (multidrug-resistant protein) gene is knocked out. Replacement of the mMrp2 gene with its human ortholog, hMRP2, functionally compensates for the loss of mMrp2 in humanized mice.
Genetic Humanization Applications
Genetic humanization has several potential applications. Among them are mimicking human drug metabolism in rodents, enhancing the predictive power of drug candidates in the research pipeline, and developing improved models of human physiology and pathology.
Despite the high conservation of the general mechanism by which foreign substances are metabolized, there are often significant differences in drug metabolism in mice and humans, including variations in receptor binding affinity and the specific effects of activated pathways. Humanizing important protein components of the drug metabolizing system (i.e., xenobiotic receptors, cytochromes, and drug transporters) thus provides a mouse model for human metabolism of foreign substances that can be used to predict metabolite formation, pharmacokinetics, and drug-drug interaction in an in vivo system that closely resembles the human situation. (Figures 1 and 2) Complex (multiple) humanized mouse models developed by Taconic have been successfully used for these purposes.4-11
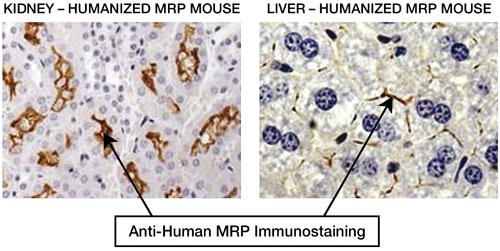
Figure 2. Immunohistochemistry of kidney and liver from humanized mice expressing hMRP. Results demonstrate correct tissue and intracellular expressing of hMRP.
Increase Human Specific Drug Candidate Predictability
Humanized models are particularly useful for testing human-specific drug candidates such as therapeutic antibodies or other biologics in a preclinical setting, thus increasing the predictive power of efficacy studies. For example, a top five pharmaceutical company recently demonstrated that an inhibitor of mPGES1—an enzyme whose activation results in symptoms associated with inflammatory pain—effectively reduced pain in a preclinical humanized mouse model after it had been deemed ineffective in a mouse model that only expressed murine mPges1.12 By replacing murine mPges1 with its human ortholog, Taconic was able to provide an appropriate and efficient tool to test this human-specific compound in vivo.
Humanization for Physiology Mechanism Clarification
Humanized mouse models also help clarify specific mechanisms of human physiology in both normal and pathological states. One study employing genetically humanized mouse models generated by Taconic provided support for the mechanism underlying alcohol addiction in men.13 Specifically, a single nucleotide polymorphism (SNP) in the μ opioid receptor identified in a genome-wide association study was found to be linked with alcohol addiction in males, with the normal A nucleotide being replaced with a G nucleotide when addiction is present. To phenotypically support the functional relevance of this SNP, genetic humanization was used to mimic the human genotypes in mice by exon swap, such that the human AA variant was produced in one group of mice (wild type), and the human GG variant was in another group (mutation).
Both humanized mouse models were used to test the relevance of the human genetic variations of this gene in alcohol addiction in vivo. When the mice were fed alcohol, the animals carrying the mutation demonstrated more dopamine release, as measured by microdialysis. As dopamine release has an established role in reward processing, this finding has provided a potential mechanism by which a specific mutation enhances reward perception in response to alcohol in men, thereby increasing the chances of developing alcoholism. Incorporated into such studies, humanized mouse models can provide information far beyond the mere association of genes and certain diseases. Going further, humanized models can help scientists determine how genes mediate pathological processes.
Conclusions
As a result of international focus on genome sequencing, increasingly more human genetic variations are being identified. The importance of clarifying the relevance of such variations is thus becoming more apparent.
Humanized mouse models provide tools for pinpointing the function of such genetic variations and for elucidating the mechanisms by which these genetic variations give rise to specific phenotypes. They are thus invaluable for investigations aimed at understanding human disease and its response to therapeutic interventions.
To learn more about how humanized mouse models can impact your research outcomes visit: www.taconic.com/gen
Taconic
Ralph Gareus, Ph.D.
Associate Director
[email protected]
www.taconic.com/gen
Note: References for this article have not been included due to size limitations.
Please visit www.taconic.com/genrefs for a complete list of references.


