October 15, 2013 (Vol. 33, No. 18)
Australian Firm Looks to Commercialize Second-Generation Compounds Obtained from Isis
So far, 2013 has been a good year for antisense technology. In fact, it’s been great.
In January the FDA OK’d Isis Pharmaceuticals’ Kynamro™ (mipomersen sodium), the only currently marketed antisense drug. It’s for patients with homozygous familial hypercholesterolemia.
This was the second antisense approval for Isis, whose Vitravine was the first to be given the green light in 1998 for cytomegalovirus retinitis. But the company discontinued the drug in 2004 due to an evaporating market.
Earlier this month, Isis signed their fourth collaboration with Biogen Idec in the past two years—a six-year, up-to-$320 million-plus partnership designed to develop new therapies for neurological diseases.
Watching all this, anything but impassively, is Antisense Therapeutics (ATL). Founded in 2001 by Circadian Technologies, in collaboration with Isis, Australia-based ATL made the strategic decision early on to in-license second-generation antisense leads from Isis. The company owns worldwide exclusive licenses to these drugs for all disease applications. In exchange for these licenses, Isis receives a share of the proceeds and income from ATL’s commercialization of the drugs
“The company selected its pipeline of antisense drugs by targeting diseases where there is a clear need for improved therapies and where the known characteristics of antisense drugs (i.e. potency, long tissue half-life, unique tissue distribution, good tolerability, and the potential for convenient subcutaneous dosing) provide therapies with important clinical advantages over existing therapies,” Mark Diamond, managing director, told GEN.
Diamond believes the company has further de-risked its development strategy by working on antisense drugs directed to clinically validated biological targets, thereby reducing target risk.
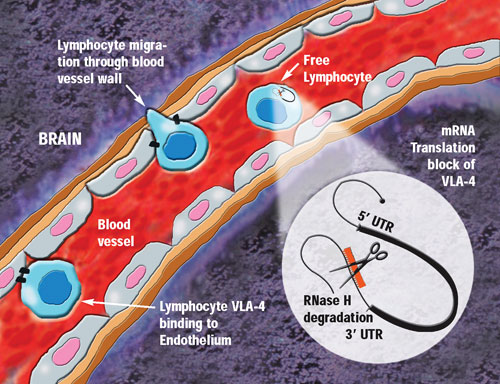
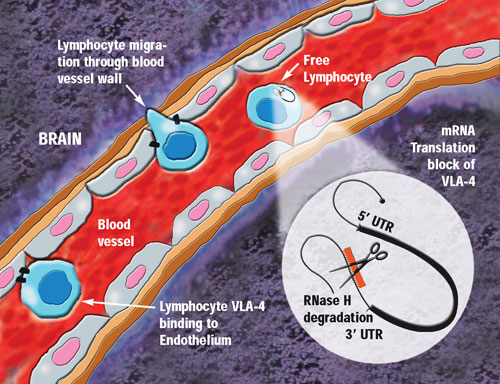
Lymphocyte migration into the brain is reportedly prevented by antisense VLA-4 inhibition.
Modus Operandi
ATL’s method of operating is to focus on taking licensed compounds through preclinical development and into the clinic. The ultimate goal is to outlicense products to larger companies while potentially taking at least one drug all the way to market.
“We have exclusively licensed three drugs from Isis and licensed patents directed to the antisense drug platform and manufacturing patents,” continued Diamond. “We are responsible for all the R&D, which we plan in-house and contract out to experienced practitioners. We select the best sites to undertake these activities on a global basis, which we project manage from our offices in Australia.”
The company has preclinical and clinical studies and development activities currently running in Australia, Europe, the United States, and China.”
One of the main advantages of antisense therapy, according to Diamond, is the ability to move relatively inexpensively and efficiently into clinical development, with platform-based advantages over existing treatments that are built into ATL’s programs from the start.
“Unlike conventional drug discovery, the process for producing an antisense lead inhibitor is rapid, once the biological target is identified,” he explained. “Many antisense sequences can be generated to its genetic sequence and tested in vitro to confirm drug potency and then studied in animals to confirm activity and safety.”
Producing an antisense drug compound for preclinical and clinical development is a relatively simple and inexpensive procedure using essentially the same chemical processes for all antisense drugs, claimed Diamond, adding that “this compares to the manufacture of a small molecule, peptide, protein, or monoclonal antibody drug, which requires a workup for each individual drug.”
As a result of the clinical experience with antisense compounds in general, ATL appears comfortable enough with their safety profiles to try to position its drugs against compounds that have less attractive safety profiles.
Also, compared to protein-based drugs, ATL expects antisense drugs to be less expensive to manufacture on a commercial scale. “The scaleup for commercial quantities of antisense drugs is well established, and there are several large-scale manufacturers,” said Diamond. “This again is helpful when considering protein-based drugs as your main competitor.”
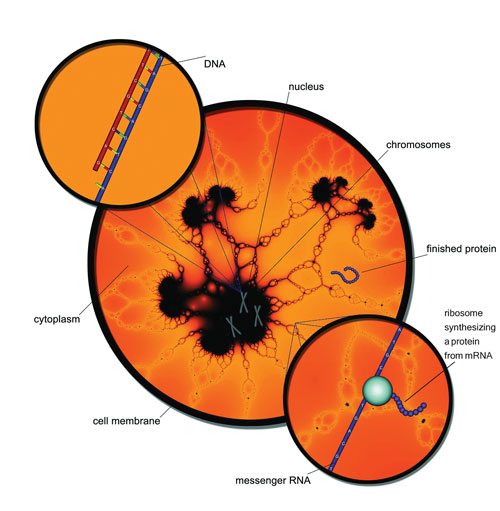
Protein production process shown inside a cell.
Products in Development
Currently, ATL is overseeing research on three drugs licensed in from Isis. ATL1102 (injection), a second-generation antisense drug to VLA-4, has shown “outstanding activity in Phase II studies potently reducing new active brain lesions in multiple sclerosis patients,” explained Diamond. It also has the potential to assist in the release of hematopoietic stem cells for their collection from the blood for use in the treatment of cancer, he added.
“We are, however, particularly keen on our drug ATL1103, which is an antisense inhibitor of the growth hormone receptor (GHr). This drug is in a Phase II clinical trial currently under way in Europe for the treatment of acromegaly,” continued Diamond. “Interim data from the trial is anticipated at the end of this year.”
Diamond pointed out that GHr is expressed in the liver, which is an ideal target tissue for antisense drugs as they accumulate in the liver at high concentrations. Several antisense drugs in the Isis pipeline are directed to targets expressed in the liver, and have demonstrated potent pharmacodynamic and pharmacological effects, he added.
The company also views acromegaly as a smart first-up lead indication, before expanding into cancer treatment.
“Acromegaly is an orphan drug disease, and orphan drug development is supported by development, regulatory, and IP incentives, which makes it attractive and affordable from a development perspective,” Diamond pointed out.
The company is also very satisfied with the endpoint for treating acromegaly, which is normalization of serum IGF-I (sIGF-I).
“Acromegalics have a tumor of the pituitary gland which causes them to produce too much growth hormone and, in turn, excessive amounts of IGF-I in the blood that leads to all the side-effects and significant morbidity and mortality associated with the disease,” said Diamond.
“sIGF-I is an easy endpoint to measure. It is also able to be assessed through preclinical and clinical development. sIGF-I normalization is also the endpoint for regulatory approval of acromegaly therapies. So we have the same, easy-to-measure endpoint, through all stages of development.”
He added that the company has shown that ATL1103 reduces sIGF-I in all its preclinical animal studies in mice and monkeys to date, and there’s been a preliminary indication of activity with reductions of sIGF-I in its Phase I normal volunteer study.
Other products in the ATL pipeline include ATL1102 (inhaled), which is in preclinicals as a potential treatment for asthma, and ATL1101, also in the preclinical stage, where it is being investigated as a potential therapy for cancer.
Looking ahead to the next 5 to 10 years, Diamond expects ATL to successfully commercialize the drugs in its current pipeline through successful licensing and/or joint development arrangements.
“As highlighted earlier, we also have the goal of taking one of these drugs all the way to market ourselves,” he said. “This would likely be for an orphan drug indication, and our current best prospect for this is ATL1102 for stem cell mobilization.”
Antisense Therapeutics
Location: 6 Wallace Avenue, Toorak, Victoria, 3142, Australia
Phone: 61 (3) 9827 8999
Website: www.antisense.com.au
Principal: Mark Diamond, Managing Director
Number of Employees: 4
Focus: Antisense Therapeutics is involved in the development and commercalization of antisense drugs based on second-generation antisense chemistry in-licensed from Isis Pharmaceuticals.