March 15, 2012 (Vol. 32, No. 6)
Personalized medicine made significant strides in 2011 with two new targeted cancer therapies, the FDA release of its companion diagnostics draft guidance, and routine clinical genotyping for some oncology applications. However, many challenges remain. An estimated 90% of currently marketed drugs are effective in only ~40% of patients annually, costing ~$350 billion for medications that don’t work.
Hope may be on the horizon, though, bolstered by new successes and novel approaches in biomarker analyses. GTC’s “Oncology Biomarkers” conference will feature pointed discussions about overcoming challenges to translation of biomarkers to the clinic. It will also showcase emerging technologies for assessing biomarkers, including the use of isogenic cell lines to stratify patient populations, next-generation sequencing for mutational analysis, and identification of unique biological targets.
Over the past few years there has been a clear shift in oncology from traditional cytotoxic agents to more molecularly targeted drugs, says Adam Schayowitz, Ph.D., director of business development, BioMarker Strategies.
“The challenge is how to match patient biomarkers with anticipated drug response. Often a drug is developed for a given indication, without knowing in which subset of patients it will be most effective.”
Part of the bottleneck is the way tumor specimens are processed. “Typically, when biopsies are obtained, tissue is fixed using an approach that was developed in the 19th century (FFPE sections). Then static biomarker tests are performed using methods such as immunohistochemistry, DNA sequencing, and DNA amplification.
“The problem with this approach is that this tissue is dead and unable to be interrogated for true functional value. As such, what actually happens within the signal transduction network of living cells is lost.”
BioMarker Strategies believes one way to bridge the chasm between biomarkers and drug response is by developing a new paradigm for testing tumor specimens.
“A better approach,” Dr. Schayowitz suggests, “is to keep the cells alive long enough to obtain a functional profile that would reveal information about the signal transduction networks that predict patient response to targeted drug therapy.
“Our company has developed an ex vivo biomarker testing system called SnapPath™ to enable this approach. It is an automated live tumor cell processing platform that allows us to characterize how a patient’s live tumor cells respond to pathway stimulants (e.g., growth factors) or inhibitors (targeted drugs).”
Initially, a fine-needle biopsy harvests cells from the patient’s tumor. The sample is then placed in a ready-made cartridge that can be processed in the self-contained instrument. The automated processing takes less than 30 minutes and occurs in steps.
First cellular aggregates are mechanically dispersed. Nontumor material is depleted, and the purified cells are distributed in up to four chambers. While some cells are untreated and remain at baseline, others are treated with various stimulators and inhibitors (therapeutic drugs). Finally, samples are lysed and stabilized for off-platform extraction and analysis.
“The idea is to obtain functional signaling profiles of the phosphoprotein signaling networks to compare the treated and untreated malignant cells. SnapPath is amenable to analytic technology procedures that can also be performed for RNA and DNA analysis. We are currently conducting clinical studies at a growing number of cancer centers. While we are starting with advanced melanoma, the SnapPath platform is designed to process all solid tumors.”

BioMarker Strategies’ SnapPath™ system incorporates an automated, live-tumor-cell processing device with first-in-class, functional, ex vivo biomarker tests to improve drug development and inform clinical decision-making for targeted cancer therapeutics. SnapPath stimulates a patient’s live tumor cells—outside their body—to obtain a Functional Signaling Profile of the signal transduction network that is not possible using static, genomic biomarkers from dead, fixed tissue.
Isogenic Cell Lines
Transforming biomarker data into clinically relevant companion diagnostics requires overcoming several key challenges, says Andrew Grupe, Ph.D., senior director of pharmacogenomics discovery research, Celera, a business of Quest Diagnostics.
“One hurdle is regulatory issues that inevitably arise. These require peer guidance by collaborating with experts who can help transition biomarker assays from research to diagnostic. Second, one needs to address reimbursement issues. In order for tests to be reimbursable, there must be proven clinical utility. For example, we are working with Medco to determine whether genetic testing could impact compliance of patients on statins.
“A third key aspect for transitioning biomarkers to the clinic is the requirement point to provide solid confirmatory evidence for the utility of specific biomarkers. Also, associated clinical trials must be powered sufficiently. For example, biomarkers may be identified or confirmed in properly powered Phase II trials and then need to be tested in a Phase III trial with a prespecified hypothesis that spells out specifics of anticipated results.”
According to Dr. Grupe, the use of isogenic cell lines can be a powerful means for assessing biomarkers that help stratify patient populations.
“Isogenic cell lines are engineered or selected to model human cancer genotypes, i.e., a specific patient population. These are created using homologous recombination techniques that knock-in or knock-out disease causing mutations. Studies are performed comparing these engineered lines with wild-type isogenic cell-line controls. Isogenic cell lines provide an important strategy to assess cancer treatments in the context of somatic mutations.”
Since hundreds of cancer genes have now been characterized, it is possible to examine specific genetic mutations and evaluate how these interact with the rest of the cellular apparatus to modify patients’ responses to specific treatments. For example, mutations in K-Ras, BRAF, and PI3K may impact response to cancer drugs.
“We’ve developed expression profiles of individual genes and multigene signatures that give us information related to treatment efficacy. We have identified gene responses to tamoxifen treatment for breast cancer and tested the signatures in isogenic lines. Our isogenic cell-line results demonstrated that the signature can predict results consistent with genetic background.”
Autoantibody Biomarkers
Autoantibodies are present in many conditions such as cancer, autoimmune disorders, and transplant rejection. This affords an opportunity to develop diagnostic and prognostic assays, as well as to monitor disease progression and treatment effectiveness. There are several challenges, however.
“Only a fraction of patients develop autoantibodies to a given protein,” indicates Lisa Freeman-Cook, Ph.D., senior manager, R&D, Life Technologies.
“Thus, identification of multiple biomarkers in patient samples is important. We have developed functional protein microarrays (ProtoArrays®) that may be used to screen complex biological mixtures such as serum to detect autoantibodies directed against any of the 9,000 proteins on the microarray.”
The quality of the protein on the array is an important factor. “The ProtoArray contains proteins that are expressed in insect cells and purified under native conditions. These more closely recapitulate appropriate post-translational modifications and allow a more native protein conformation.”
Dr. Freeman-Cook says the utility of the ProtoArray for identifying biomarkers was demonstrated recently by investigators looking for novel autoantigens in rheumatoid arthritis, prostate cancer, and Alzheimer disease. But the next step is validation of identified biomarkers.
Validation studies typically assess candidate markers against an increasing number of independent patient samples. Thus throughput becomes important along with accurate measurements of sufficient sensitivity, dynamic range, and reproducibility to differentiate responders from nonresponders.”
Since the ProtoArray was not designed for high throughput, the company decided to employ Luminex xMAP technology.
“We developed the ProtoPlex™ Immune Response Assay featuring Luminex xMAP technology. No one before has paired these two technologies. The idea is to first utilize the ProtoArray to discover biomarkers of interest. Then, Luminex technology can be used for validation.”
The Luminex xMAP technology employs spectrally distinguishable fluorescent beads to which antigens of interest are coupled. “Cross-reactivity issues have not been a problem. As a result, we are able to accomplish higher levels of multiplexing (>80-plex) per assay with any antigen. We can process up to 500 samples per day with very low CV’s (
The company markets ProtoArray products and provides ProtoArray and ProtoPlex custom services. “We feel that combining these two technologies now not only supports biomarker discovery for a wide variety of diseases, but also allows validation with the familiar Luminex technology,” Dr. Freeman-Cook remarks.
Life Technologies’ ProtoArray® Human Protein microarrays, containing more than 9,000 purified full-length human proteins, can be used to identify autoantibody biomarkers from blood for a variety of diseases. These biomarkers have applications in disease diagnosis or prognosis or in monitoring treatment efficacy, according to the firm. Once identified, the autoantibody biomarkers can be validated using the ProtoPlex™ Immune Response Assay featuring Luminex® xMAP technology.
Noninvasive Assays
Most biomarker analyses are generated by excising tumor samples from patients. A noninvasive means, however, is assessing biomarkers in body fluids, such as urine. One drawback to the use of body fluids is the low concentration of analytes in the sample, notes Anthony P. Shuber, CTO, Predictive Biosciences.
“Biological fluid-based assays can be very powerful. But the presence of biomarkers at very low levels has been a hindrance. To date, there have been no biological fluid-based diagnostic tests that have sensitivity comparable to tissue-based assays.”
According to Shuber, Predictive Biosciences is utilizing next-generation sequencing to detect biomarker mutations.
“We have been focusing on mutations in the fibroblast growth factor receptor three (FGFR3) gene, which are present in 30–50% of bladder cancer patients. The presence of FGFR3 mutations correlates with a lower rate of bladder cancer recurrence. Next-gen sequencing as a diagnostic platform has a great deal of appeal, because it allows sensitivity greater than qPCR.”
The company collects a small sample of urine from patients and then subjects it to next-generation sequencing to determine the presence of mutations.
“No one really knows what percentage of mutated DNA exists in body fluid, although small amounts of 1% have been detected. We have developed a new urine-based assay that uses ultra-deep next-generation sequencing on amplicon-generated FGFR3. We found that this assay reliably detects a mutation when it is present in as little as .02% of the total amount of DNA in urine.”
Shuber says it is unlikely that every clinical lab will make the investment in a next-generation sequencing apparatus. “Where this is likely to go is via samples obtained at a point of care and sent to a lab that specializes in next-gen sequencing. Clearly, there is a place in the clinical diagnostic laboratory for next-gen sequencing. We are certainly moving in that direction.”
More than 250,000 women are diagnosed in the U.S. with breast cancer every year. A majority of patients (~170,000) are estrogen receptor positive (ER+), and subsequently receive a regimen that includes anti-estrogen therapeutic agents such as tamoxifen or aromatase inhibitors.
Novel Cancer Biomarker
“Unfortunately, more than 40% of such women are not responsive or become nonresponders to these treatments,” says Ginette Serrero, Ph.D., CEO, A&G Pharmaceutical. “There is an unmet need to identify protein-based biomarkers for anti-estrogen resistance to be run alongside tests used in the standard of care that could better predict who will and who won’t respond to treatment.”
A&G Pharmaceutical has identified one such biomarker called GP88. “This is an autocrine growth and survival factor produced, secreted, and absolutely required by the cancer cells. A&G has performed studies demonstrating that this biomarker plays a critical role in cancer development, invasiveness, and for the survival of breast and lung cancer.
“We have developed two diagnostic kits that measure GP88 in tumor tissue and in serum of patients. The tissue test has been validated in two independent clinical trials. These demonstrated that in ER+ breast cancer patients, an elevated GP88 was correlated with a fourfold increase in the risk of disease progression. Further, ongoing prospective clinical studies have found that serum GP88 levels are elevated in breast and lung cancer patients as compared to healthy subjects.”
Dr. Serrero also described the company’s development of a companion therapeutic. “We have developed a neutralizing monoclonal antibody against GP88 that is undergoing preclinical development. Our studies include mouse xenograft models of tamoxifen resistance. We found that when used together, the monoclonal antibody potentiated tamoxifen response.”
Leading investigators in the field suggest that within the next five years, oncologists and their patients will have many more therapeutic options. Perhaps as many as 25 molecularly targeted agents for cancer therapy will be available.
Within this time frame, virtually every possible mutation will have been identified in the genomes of the most common cancers. This will allow increasingly effective monitoring of the treatments for cancer by examining robust biomarkers from samples obtained by minimally invasive means.
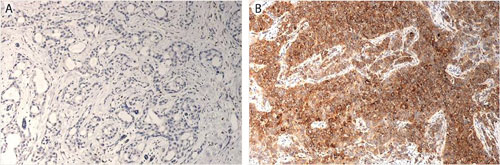
Micrographs of breast cancer lesions (A) low-risk, GP88 negative, and (B) high-risk, GP88 positive, invasive ductal carcinoma [A&G Pharmaceutical]
Chemokines and Cytokines as Potential Markers for Personalizing Leukemia Treatment
“Leukemic cells don’t live in isolation; they’re interacting with their environment within the bone marrow, they’re interacting with their environment in terms of the blood that flows by them,” explains Steven Kornblau, M.D., professor at MD Anderson Cancer Center.
Dr. Kornblau is working to understand how the cytokines and chemokines that the leukemic cells might be getting exposed to also affect protein expression. The idea that researchers in his lab might find different cytokine and chemokine expression patterns within leukemic cells led to determining ways to profile large numbers of cytokines.
The researchers considered using ELISA kits for the chemokine and cytokine studies but realized that this approach would require a significant amount of sample, plus there was concern about the impact of batch effects on results.
“We were looking for something where we could analyze a lot of cytokines and chemokines simultaneously that also wouldn’t require too much material,” Dr. Kornblau notes. “Around that time we saw that Bio-Rad (www.bio-rad.com) was creating large cytokine kits, where you could look at numerous chemokines and cytokines simultaneously.”
The lab used the Bio-Plex Pro™ human cytokine 27-plex assay to study a large panel of serum samples from acute myelogenous leukemia (AML) and myelodysplastic syndrome (MDS) patients (178 AML and 114 MDS samples). Because MDS is often considered to be “preleukemia,” one of the questions to be addressed was whether MDS and AML exhibit similar or unique cytokine patterns.
“The first thing we found was that for both diseases, the pattern of expression was markedly different from the 19 normal controls that we had included,” notes Dr. Kornblau. “The second thing we found was that for 24 of the 27 cytokines, there really wasn’t a difference between their expression in MDS and their expression in AML.”
Analysis of data generated in this study led to segregation of patient samples into eight different signatures. Identification of these signatures led to their correlation to different clinical leukemic features such as cytogenetics, outcome, and remission duration.
“We found to our surprise that there tended to be a good, an intermediate, and an unfavorable group of signatures,” says Dr. Kornblau. “These were independent of other established clinical features like cytogenetics, age, or like having an antecedent hematological disorder. We were surprised to see that the cytokine and chemokine profiles actually were prognostic on their own.”
The results of this research set up the idea going forward that chemokine and cytokine patterns can be used to inform different therapies and enhance the ability of clinicians to affect leukemic cell growth or eliminate the resistance of those leukemic cells to different therapies already in use, according to Dr. Kornblau.



