March 15, 2011 (Vol. 31, No. 6)
Experts Now View Process Analytic Technology as a Critical Component of Quality-by-Design
Nearly eight years after the U.S. FDA’s landmark guidance on process analytic technology (PAT), implementation remains slow and uneven, leading some to ask when this initiative will achieve the broad improvements originally promised.
After an initial flurry of articles from major drug firms, and countless conferences devoted to PAT, implementation seems to have barely begun, and the technologies employed do not inspire awe. “Too many groups are still just thinking about it,” observes Justin Neway, Ph.D., CSO at Aegis Analytical.
PAT has probably made a difference in improving process understanding and product quality, but Dr. Neway admits that “not enough hard data is publicly available to know for certain.” More useful, he believes, has been wider adoption of quality-by-design (QbD) principles, which work by “leveraging existing data without spending money and time on real-time instrumentation that may not be needed.”
FDA did not respond to interview requests on the effectiveness of its PAT initiative.
Fits and Starts
Some PAT projects appear promising during process development, but never make it past that point. Patricia Andres, Ph.D., director of drug development at contract development firm SSCI, uses measurements of turbidity, temperature, and Raman analysis in designing crystallization processes for active pharmaceutical ingredients, but is unaware of analytic technology being passed down to manufacturing. “I believe that technology transfer to manufacturing may be an issue,” she admits. “It would take a lot of effort for something that would only be used a few times.”
Emil Ciurczak, who heads Doramaxx Consulting, a firm specializing in near-infrared analytics, says that even success of individual projects does not guarantee that PAT becomes part of the pharma/biotech DNA. He observes that even companies that reduce manufacturing times substantially by implementing analytics often drop the ball with later projects.
“They’re afraid of seeing what they’re going to see, of what’s really wrong or can go wrong with their process,” he explains. “They’d rather shoot craps and hope nothing shows up.”
Then there’s the regulatory uncertainty angle, which has plagued PAT since its original promulgation in 2003. “Manufacturers are still quite wary about adopting new technology and having to document it to regulators. It happened during the 1970s with HPLC, and it’s happening now with PAT,” Ciurczak says.
Speaking of HPLC, the workhorse analytic tool of pharma/biotech labs has barely made inroads onto the manufacturing floor despite several articles per year demonstrating its feasibility. The knock on chromatography is its slowness and complexity. John Hanrahan, Ph.D., CTO at silica particle maker Glantreo, believes these criticisms are less and less justified as HPLC systems become faster.
“We now have the capability for manufacturing small, porous particles that could be used in high-pressure, and rapid, HPLC,” Dr. Hanrahan is talking about particles that are significantly smaller than the sub-2-micron silica used in rapid HPLC techniques today, for example smaller than 1 micron. These will require pressures well above the 20–25 kpsi that the best pumps deliver today and could conceivably return results in a matter of seconds.
But as Dr. Hanrahan notes, no significant process today employs HPLC in a PAT environment. Manufacturers who are unimpressed with generating a diagnostic HPLC trace every few minutes are unlikely to be won over by a 50% or even 90% reduction in analysis cycle time.
Positive Signs
Since current analytics and controls do not necessarily indicate product quality, interest in deploying PAT tools closer to the production environment will continue, believes Sam Watts, Ph.D., business development officer at Stratophase. “But the complexity of analytical methods, with respect to data-gathering and subsequent analysis and interpretation, leads to significant challenges in applying these techniques to multipoint, real-time, and inline applications.”
Stratophase produces optical microchip sensors that monitor both process status and product quality in real time, or which serve as a gateway for triggering process sampling and slower, but more detailed off-line analysis. Stratophase has worked closely with a number of process experts in a range of industries on PAT-type initiatives, including manufacturers of pharmaceuticals and precursors. “A number of our demonstration systems are now being deployed with key end users, which will be critical for our product-development activities.”
Dr. Watts believes there is a general trend toward accepting “novel monitoring solutions” that present a compromise between the high chemical specificity associated with historical analysis and rapid, scalable methods. There is the potential for a combined solution as well, employing both full-featured analysis and real-time multipoint monitoring to trigger the more in-depth analysis.
Stratophase has worked with GlaxoSmithKline using Stratophase’s temperature compensated refractive index measurement to determine deviation from ideal feedstock concentration within a continuous flow reactor. It has also conducted trials monitoring fermentation processes for alcoholic beverages, as well as in the manufacture of biofuels, biopolymers, and biopharmaceuticals.
The firm is building an empirical model for E. coli fermentation that will compare real-time process status against a model. “The validation of this model-building process is a key stage in our development of PAT-type bioprocess applications,” says Dr. Watts.
Lyophilization is a classic example of a process for which quality has traditionally been “tested in,” and where PAT could help considerably.
“Smart” lyophilization technology, licensed from the University of Connecticut and commercialized by SP Scientific is slowly helping to change the optimization of primary freeze-drying, that is the removal of all free ice.
“Over the years, our understanding of lyophilization technology has evolved from an art, or black box activity, to a science,” says Leslie Mather, director of pilot lyophilizers at SP Scientific’s Stone Ridge, NY, facility.
The technique, which incorporates process analytics during development and optimization, involves doing some preliminary work to understand the thermal properties of the formulation through differential scanning calorimetry and freeze-drying microscopy. This leads to an understanding of how the drug freezes and melts, and helps establish boundaries such as minimum product surface area.
Then, during the first—and only—experimental run, the equipment takes very rapid pressure measurements during drying. “The valve between the product chamber and condenser must close in less than one second,” Mather says. A pressure reading is then recorded over the next 25 seconds, after which the valve is re-opened.
According to Mather achieving the optimal lyophilization process takes just one experimental run.
Proprietary algorithms translate pressure data into values for product resistance, temperature at the ice surface, shelf temperature, heat flow, mass transfer, and ice thickness. The resulting optimization cycle enables operators to conduct lyophilization in walk-away mode. Although intended only for optimization, the technique may be employed during manufacturing and as an add-on.
Engineers always model biomanufacturing processes to identify bottlenecks and perform general streamlining before any equipment is assembled. Rafiqul Gani, Ph.D., professor of chemical and biochemical engineering at the Technical University of Denmark, goes a step further and models the process from the perspective of suitable analytics.
Theoretically a PAT model can be as useful as the process model itself. Armed with a product and process, Dr. Gani’s model analyzes the steps and equipment required, identifies variables that are most critical for monitoring product quality, and recognizes which of these should be measured and monitored as well as equipment, cost, and time for deployment.
Thus far, working through an industrial consortium, he has applied his PAT model to fermentations, milk pasteurization, tablet manufacturing, and crystallization processes.
“We think that, as in other situations, a PAT model will not directly replace experiments, but supplement it,” Dr. Gani says. “The more information one has, the better the analysis and decision making.”
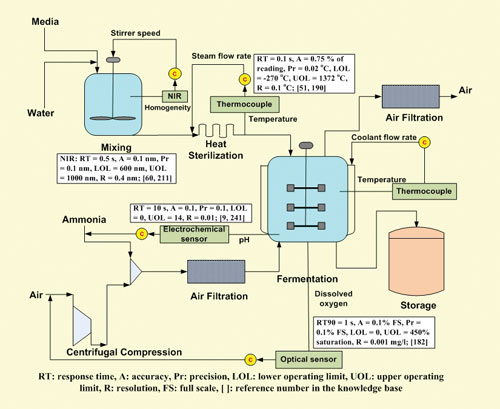
Schematic of the fermentation process after addition of a designed PAT system [Rafiqul Gani, Ph.D., Technical University of Denmark]
The Usual Suspects
Dr. Watts agrees that adoption of PAT in pharmaceuticals has been slow, and for good reasons. He believes that the primary drivers for PAT are (or should be) economic rather than regulatory. Thus, the industry will adopt PAT only if the methodology “enables either significant improvements in production yields or access to processing regimes that would not normally be achievable without PAT.” This is one reason, he argues, why PAT is rarely adopted retrospectively, to existing processes.
Second, he feels that “a large proportion of the PAT community has approached the problem with a relatively narrow view of analytical techniques, optimistically believing that the ideal route forward is to deploy the laboratory-based technologies that they have historically used.” This has proved to be a challenge, he says, since the complexity of data-gathering and analysis often leads to issues with gaining real-time contextual information. “And the cost associated with deploying such equipment on the scale required is also proving prohibitive.”
These factors explain why PAT is only beginning to make its mark in pharmaceutical manufacturing. PAT and QbD are philosophies, which, Dr. Watts admits, have seen widespread adoption in other process industries. Which is why he thinks PAT’s rise in pharmaceutical manufacture is inevitable. “However, the rate of adoption in a historically conservative industry is difficult to predict and not helped by the current economic climate, but I do believe the future looks bright.”
According to John Dubczak, director of operations for endotoxin and microbial detection at Charles River, the pharmaceutical industry has been slow to accept new technologies due to validation and regulatory requirements. This is ironic, he says, because “PAT is supposed to facilitate innovation and the regulatory process as well.” Instead, many processes remain “frozen” within a testing-in-quality paradigm.
One of the hurdles has been re-tooling analytic methods to operate in real- or near-real time. The shortcomings of traditional analytic instrumentation in this regard, e.g., HPLC, are well known.
Even less well characterized are biochemical tests that are critical to keeping a process moving. One such test, for bacterial endotoxins, the Limulus amebocyte lysate (LAL) assay, has been around for years but takes several hours in its traditional format.
Charles River has significantly revamped the LAL assay through its Endosafe®-PTS, a rapid, quantitative, point-of-use test system based on an enzyme harvested from Limulus polyphemus (horseshoe crab). Many experts now view PAT as one of several critical components of quality by design (QbD), specifically the function by which critical quality parameters are recognized (through design of experiment) and measured on the manufacturing floor. But PAT by itself does not guarantee that the correct parameters and values will be applied. “It’s like cruise control,” comments Ciurczak of Doramaxx. “If you apply cruise control at an illegal speed you’ll still get a ticket.”
“There will always be conference sessions devoted to PAT, but the emphasis has definitely shifted to QbD,” adds Dr. Gani. In part, this is related to the difficulty in getting companies to adopt PAT methodology or, in Dr. Gani’s case, PAT modeling approaches. “All major companies have PAT groups, and budgets for buying equipment, but I’m not entirely sure if they’re actually applying it in ways that regulators intended. All things considered, QbD has a better chance of showing results.”
Charles River is attempting to make it easier for companies to adopt new technologies, a PAT imperative. The company recently retooled the LAL bacterial endotoxin assay using its Endosafe-PTS, a rapid, quantitative, point-of-use test system.