Kim D. Anderson The University of Miami Miller School of Medicine The Miami Project to Cure Paralysis
James D. Guest The University of Miami Miller School of Medicine Miami Project to Cure Paralysis and The Neuroscience Program
W. Dalton Dietrich The University of Miami Miller School of Medicine The Miami Project to Cure Paralysis and The Neuroscience Program
Mary Bartlett Bunge The University of Miami Miller School of Medicine The Miami Project to Cure Paralysis and The Neuroscience Program
Rosie Curiel The University of Miami Miller School of Medicine
Marine Dididze The University of Miami Miller School of Medicine The Miami Project to Cure Paralysis
Barth A. Green The University of Miami Miller School of Medicine The Miami Project to Cure Paralysis
Aisha Khan The University of Miami Miller School of Medicine The Miami Project to Cure Paralysis
Damien D. Pearse The University of Miami Miller School of Medicine The Miami Project to Cure Paralysis and The Neuroscience Program
Efrat Saraf-Lavi The University of Miami Miller School of Medicine
Eva Widerstrom-Noga Bruce W. Carter Department of Veterans Affairs Medical Center The Miami Project to Cure Paralysis and The Neuroscience Program
Patrick Wood The University of Miami Miller School of Medicine The Miami Project to Cure Paralysis
Allan D. Levi M.D., Ph.D. The University of Miami Miller School of Medicine The Miami Project to Cure Paralysis
Following Spinal Cord Injury, Schwann Cell Transplantation Is Promising
Cell transplantation is a leading candidate therapy to repair tissue damage in the traumatically injured spinal cord. Schwann cells (SCs), glial cells normally present in peripheral nerve, are responsible for axon regeneration after nerve injury. Based on their clear role in successful axon regeneration, the idea arose to test whether purified populations of SCs could mediate re-growth of axons and myelin in the damaged spinal cord, where they are not present normally. The SCs spontaneously enter the injured human spinal cord where they may contribute to limited restoration of myelin sheaths around axons.1,2 Transplanted SCs have shown the ability to survive and provide some degree of tissue repair when used in experimental paradigms of spinal cord injury (SCI) in rodents.3–6
In the lesion milieu, transplanted SCs may be effective through their production of growth promoting trophic molecules,7 their deposition of matrix molecules collagen and laminin into the extracellular space,8 as well as their expression of surface membrane cell adhesion molecules such as NCAM and L1.9 SCs support the re-growth of many types of axons,3,10–13 ensheathe and myelinate regenerating or demyelinated axons,3,4,6,10,11,14 restore axonal conduction,15,16 and in some cases may improve functional recovery in subacute4,17 and chronic5 SCI animal models. SCs can reduce secondary tissue damage, neuronal cell death, and the cavitation that occurs after contusion SCI.4,14,17,18 After transplantation into the spinal cord lesion epicenter, SCs form a hybrid tissue that consists of SCs, fibroblasts, astrocyte cell processes, axons, and extracellular matrix. Whereas they do not repair all damage and do not migrate into intact parenchyma, they mediate their effects in the injury epicenter in this modified milieu.
In studies of contusion SCI in the rat using mitogen-expanded cells, we have shown that SC transplantation at seven days after injury significantly fosters axon regeneration and myelination, improves host tissue preservation,4 and reduces cavitation4,14 in a thoracic SCI paradigm and increases numbers of preserved NeuN positive neurons rostral and caudal to the injury/graft site17 after cervical SCI. Using mitogen expanded human SCs, placed as cellular cables in SCID mice and athymic female nude rats, the human SCs survive and are capable of enhancing axonal regeneration and forming myelin after transplantation in animals with sciatic nerve transection19,20 or thoracic spinal cord transection.21,22
Thus, human SCs are promising candidates for transplantation into the injury epicenter of humans with SCI. Large numbers of autologous human SCs (ahSCs) can be obtained for implantation after a peripheral nerve biopsy followed by dissociation and expansion of their cell numbers in culture with mitogens.23 Autologous cells in general offer important safety advantages over allogeneic sources that include minimal risk of disease transfer, low risk of tumorigenicity, and the absence of immunosuppressive medication requirements.
There is no direct method to extrapolate the timing of cells delivered at seven days post-injury in rodents to an equivalent time in humans; and the definition of acute, subacute, and chronic time periods in rodents versus humans is not clearly delineated. When designing a Phase I trial involving the surgical delivery of cells into the spinal cord parenchyma during the first few months after injury, however, risk must be mitigated as much as possible. The trial design described below took into account the concomitant injuries that co-occur with acute complete thoracic SCI, the need for a nerve harvest and second spinal surgery, and the need for reliable baseline data.
On the basis of this substantial body of pre-clinical data establishing the efficacy of SC transplantation into the central nervous system, and the merits of autologous preparations, a Phase 1 clinical trial was conducted to evaluate the safety and feasibility of ahSC transplantation into the injury epicenter of six subjects with subacute SCI. The trial was an open-label, unblinded, non-randomized, non–placebo-controlled study with a dose escalation design. The primary end-point was to evaluate the safety through a one year follow-up when ahSCs were administered at one of three doses within 72 days of injury to participants with complete thoracic SCI. Determining the safety of subacutely delivered SC injections into the injured spinal cord is a critical step in enabling future trials combining additional strategies with SC transplantation.
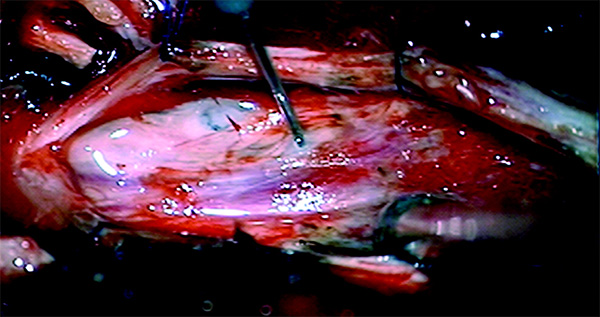
Representative images of exposed cord with needle inserted during injection. [Journal for Neurotrauma]
*Abstract
The rationale for implantation of autologous human Schwann cells (SCs) in persons with subacute spinal cord injury (SCI) is based on evidence that transplanted SCs are neuroprotective, support local axonal plasticity, and are capable of myelinating axons. A Phase I clinical trial was conducted to evaluate the safety of autologous human SC transplantation into the injury epicenter of six subjects with subacute SCI. The trial was an open-label, unblinded, non-randomized, non-placebo controlled study with a dose escalation design and standard medical rehabilitation. Participants were paraplegics with neurologically complete, trauma-induced spinal lesions. Autologous SCs were cultured in vitro from a sural nerve harvested from each participant and injected into the epicenter of the spinal lesion. Outcome measures for safety were protocol compliance, feasibility, adverse events, stability of neurological level, absence of detectable mass lesion, and the emergence of clinically significant neuropathic pain or muscle spasticity no greater than expected for a natural course cohort. One year post-transplantation, there were no surgical, medical, or neurological complications to indicate that the timing or procedure for the cell transplantation was unsafe. There were no adverse events or serious adverse events related to the cell therapy. There was no evidence of additional spinal cord damage, mass lesion, or syrinx formation. We conclude that it is feasible to identify eligible candidates, appropriately obtain informed consent, perform a peripheral nerve harvest to obtain SCs within 5–30 days of injury, and perform an intra-spinal transplantation of highly purified autologous SCs within 4–7 weeks of injury.
For the references and the full article please visit Safety of Autologous Human Schwann Cell Transplantation in Subacute Thoracic Spinal Cord Injury.
Journal of Neurotrauma, published by Mary Ann Liebert, Inc., reports on the latest advances in both the clinical and laboratory investigation of traumatic brain and spinal cord injury. The above article was first published in the November 1, 2017 issue of Journal of Neurotrauma with the title “Safety of Autologous Human Schwann Cell Transplantation in Subacute Thoracic Spinal Cord Injury". The views expressed here are those of the authors and are not necessarily those of Journal of Neurotrauma, Mary Ann Liebert, Inc., publishers, or their affiliates. No endorsement of any entity or technology is implied.