Sarepta Therapeutics is promising a rapid response to the FDA’s placing a clinical hold on the company’s Phase I/IIa trial of its Duchenne Muscular Dystrophy (DMD) gene therapy candidate AAVrh74.MHCK7.micro-Dystrophin—a month after the company trumpeted the study’s first positive results.
Sarepta acknowledged that the trial (NCT03375164) was placed on clinical hold after a “trace” amount of DNA fragment surfaced in research-grade raw material plasmid supplied by an undisclosed third-party manufacturer.
The trace fragment does not result in protein expression and is quickly cleared, according to preliminary in vivo testing cited by the company and performed by the Research Institute at Nationwide Children’s Hospital, which is partnering with Sarepta on the trial.
As a result, Sarepta said, the Research Institute and the company have developed an action plan that will include the use of GMP-s plasmid for the program, with “immediate plans” to submit it for review by the FDA.
“We understand the source of the issue, have been given clear guidance on how to address it, and have a plan to keep our clinical program on track. We are committed to holding ourselves to high standards of quality while we progress our programs with a sense of exigency,” Sarepta President and CEO Doug Ingram wrote in a letter to DMD advocates posted on the website of Parent Project Muscular Dystrophy (PPMD).
PPMD has committed $2.2 million to the Phase I/IIa trial, with support from additional Duchenne foundations and families.
On its website, PPMD noted that a series of revised draft guidances for gene therapy released earlier this month by the FDA included the agency’s intent to let gene therapy developers use Phase I/II data as pivotal data.
“We appreciate that Sarepta is taking every opportunity to understand the implications of these guidances and the most expeditious path to approval and access for our community,” PPMD stated. “We are anxious to learn more about their next steps and timeline as these conversations progress.”
No Dosing Delay Anticipated
In a statement yesterday, Sarepta said that subject to FDA acceptance of the action plan, it does not anticipate any material delay in dosing patients as originally planned by the end of this year.
“We intend to rapidly respond to the FDA’s clinical hold letter, including a commitment to the Agency to only use GMP-s plasmid,” Ingram said in the company statement. “Independently, we will also request a meeting with the Agency to discuss the micro-dystrophin program with the goal of commencing a pivotal trial by year-end 2018.”
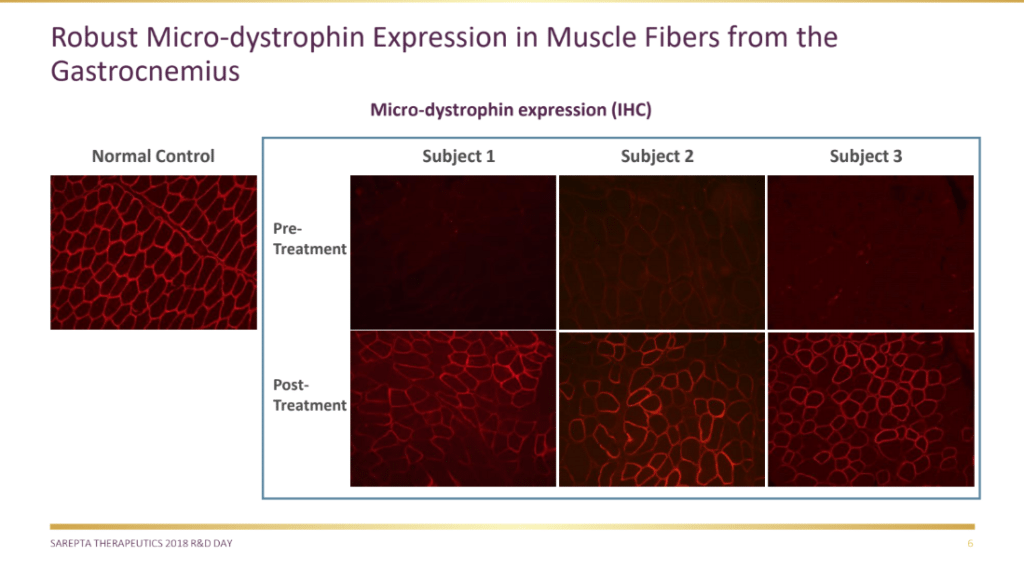
On June 19, Sarepta reported positive preliminary clinical data from the gene therapy DMD candidate.
Sarepta announced that all three patients in the Phase I/IIa study assessing AAVrh74.MHCK7.micro-Dystrophin in patients with DMD showed robust micro-dystrophin expression in muscle measured by all methods, as well as significant decreases in the levels of serum creatine kinase (CK), an enzyme biomarker strongly associated with muscle damage caused by DMD. The mean reduction of CK was over 87% at day 60.
Sarepta’s favorable results contrasted with those of another DMD therapy developer. A week after Sarepta’s positive preliminary data, Summit Therapeutics halted development of its DMD candidate ezutromid after it failed a Phase II trial—triggering a stock selloff that caused shares to lose 80% of their value.
Sarepta is one of numerous drug developers seeking to bring to market DMD therapies. Last month the FDA lifted its clinical hold on Solid Biosciences’ Phase I/II trial for its lead candidate, the gene therapy SGT-001. The hold on Solid’s IGNITE DMD study (NCT03368742) had been imposed in March after the first patient dosed with SGT-001 was hospitalized.
And in April, Pfizer said it launched a Phase Ib trial (NCT03362502) for its mini-dystrophin gene therapy candidate, PF-06939926, with early data expected in the first half of 2019.