Although scientists knew that bacteria had a sense of touch, they couldn’t quite put their finger on the underlying mechanism. But now, after a pair of studies, scientists can point to two mechanisms, each involving a different mechanosensing organ. Bacteria, it turns out, may sense surfaces either through their flagella, whip-like appendages used for swimming, or through their pili, hair-like appendages used for crawling.
When the flagella flap against a surface, or when the pili feel a tug of resistance, bacteria may begin secreting a sticky substance, allowing bacteria to become attached and begin colonizing a surface. Also, between the touch and the secretion of glue, there are chemical mechanisms, which have been explored further in the case of the mechanosensing mechanism involving flagella. Here, a second messenger within the bacteria provides an activation signal to initiate the production of a bioadhesive.
Both the flagella and the cilia studies appeared October 26 in the journal Science. The flagella study, entitled “Second Messenger–Mediated Tactile Response by a Bacterial Rotary Motor,” was led by scientists based at the University of Basel. The cilia study, entitled “Obstruction of Pilus Retraction Stimulates Bacterial Surface Sensing,” was led by scientists based at Indiana University. Both studies evaluated the behavior of Caulobacter crescentus, a bacterium commonly used in lab experiments.
“Surface-induced motor interference stimulated the production of the second messenger cyclic diguanylate by the motor-associated diguanylate cyclase DgcB,” wrote the authors of the flagella study. “This led to the allosteric activation of the glycosyltransferase HfsJ to promote rapid synthesis of a polysaccharide adhesin and surface anchoring.”
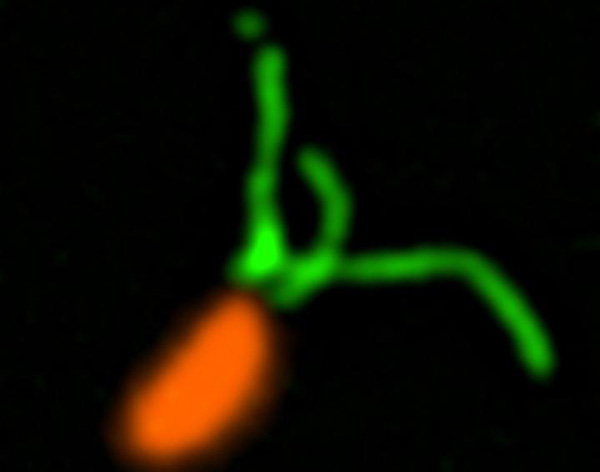
“We visualized Caulobacter crescentus pili undergoing dynamic cycles of extension and retraction,” wrote the authors of the pili study. “Within seconds of surface contact, these cycles ceased, which coincided with synthesis of the adhesive holdfast required for attachment.”
Swimming Caulobacter bacteria have a rotating motor in their cell envelope to which the flagellum is anchored. The rotation of the flagellum enables the bacteria to move in liquids. Motor rotation is powered by proton flow into the cell via ion channels. When swimming cells touch surfaces, the motor is disturbed and the proton flux interrupted.
The researchers assume that this is the signal that sparks off the response: The bacterial cell now boosts the synthesis of a second messenger, which in turn stimulates the production of an adhesin that firmly anchors the bacteria on the surface within a few seconds. “This is an impressive example of how rapidly and specifically bacteria can change their behavior when they encounter surfaces,” said Urs Jenal, Ph.D., at the Biozentrum of the University of Basel.
“Even though Caulobacter is a harmless environmental bacterium, our findings are highly relevant for the understanding of infectious diseases. What we discovered in Caulobacter also applies to important human pathogens,” added Prof. Jenal. In order to better control and treat infections, it is mandatory to better understand processes that occur during these very first few seconds after surface contact.
In the pili study, the Indiana University scientists fooled the bacteria into sensing a surface by attaching a large maleimide molecule to the pili to effectively block the hair-like structures' movement.
“It's like trying to pull a rope with a knot in the middle through a hole—the maleimide molecule can't pass through the hole the cell uses to extend and retract the pili,” said Courtney Ellison, the study's lead author and a Ph.D. student in the laboratory of Yves Brun, Ph.D., a professor of biology and the study’s senior author.
“These results told us the bacteria sense the surface like how a fisherman knows their line is stuck under water,” Brun added. “It's only when they reel in the line that they sense a tension, which tells them their line is caught. The bacteria's pili are their fishing lines.”
The discovery is possible due to the team's new method to observe how bacteria use pili to spread biofilms. They accomplished this observation with expertly delivered florescence dyes—delivered on the back of smaller maleimide molecules—that revealed the movement of these microscopic “limbs.”
“By using florescent dyes to label these microscopic structures, we're able to produce images that show the first direct evidence of the role that pili play to detect surfaces,” Brun explained.
In order to observe the movement of pili, the Indiana University team had to overcome a challenge: how to visualize the extremely thin structures and their movement. They did this by substituting a single amino acid within the chain of amino acids that comprise the pili with another amino acid called a cysteine. The maleimide, which delivered the florescent dyes to the pili proteins, binds to the cysteine. The maleimide is also the molecule used to deliver the large molecule to the cysteine in the pili protein to physically block the pili movement.
“It's like switching on a light in a dark room,” Ellison said. “Pili are composed of thousands of protein subunits called pilins, with each protein in the chain composed of amino acids arranged like a tangled mess of burnt-out Christmas lights. Swapping out a single light can illuminate the whole string.”
Engineering a cysteine molecule that could replace an amino acid in the pilins without affecting the pili's overall behavior was a major challenge, she added. The bacterium used in the experiment was Caulobacter crescentus, commonly used in lab experiments.
“We also used this method in this study to visualize the three types of pili produced by Vibrio cholerae, a bacterium that causes cholera,” said study co-author Ankur Dalia, Ph.D., an Indiana University assistant professor of biology. “Pili are critical to many aspects of Vibrio's virulence, and we are now using this powerful tool to understand how they use them.”
Next, Brun and colleagues hope to unravel precise mechanisms that link pili movement and bioadhesive production, as the two processes appear related but the exact nature of the connection remains unknown.
“The more we understand about the mechanics of pili in biofilm formation and virulence, the more we can manipulate the process to prevent harm to people and property,” Brun said.
It's estimated that biofilms contribute to about 65% of human infections and cause billions in medical costs each year. They infamously played a role in unsafe coliform levels in the water supply of 21 million Americans in the early 1990s, and, more recently, likely played a role in several outbreaks of Legionnaire's disease in Flint, MI. They also regularly contribute to global cholera outbreaks.
Biofilms cause serious damage in industry, including clogging water filtration systems or slowing down cargo ships by “biofouling” the vehicles' hulls, costing an estimated $200 billion per year in the U.S. alone. There are also beneficial biofilms, such as those that aid digestion or help break down organic matter in the environment.