![Researchers uncover the molecular pathway in brown and beige fat cells allows them to burn off calories instead of storing them as fat. [Cell]](https://genengnews.com/wp-content/uploads/2018/08/fx110166234815.jpg)
Researchers uncover the molecular pathway in brown and beige fat cells allows them to burn off calories instead of storing them as fat. [Cell]
With beach season in full swing, many folks would like the idea of having lipid cells be switched on to burn calories instead of storing them as fat. And though the idea of being able to eat that extra hot dog at the summer picnic and still fit into that bathing suit is appealing, there are even greater medical implications for understanding the pathways that govern fat metabolism. Now, investigators at Dana-Farber Cancer Institute in collaboration with scientists at the University of California, Berkeley, have identified a natural molecular pathway that enables lipid cells to burn off calories—raising the possibility of new treatments for obesity, diabetes, and other metabolic disorders.
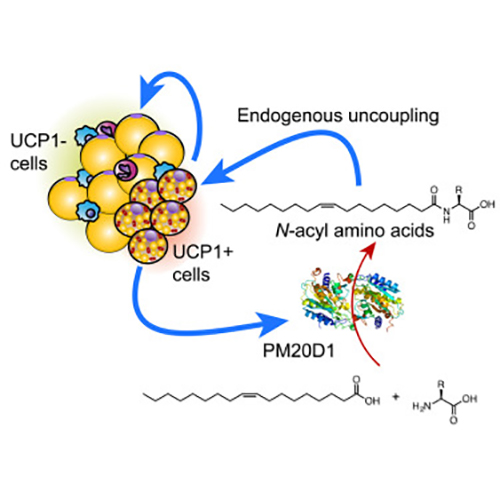
The researchers discovered the mechanism in energy-burning brown and beige fat cells in mice. Specifically, they identified an enzyme, peptidase M20 domain containing 1 (PM20D1), which was secreted by the cells and triggered the production of compounds called N-acyl amino acids. The N-acyl amino acids “uncoupled” fat burning from other metabolic processes—allowing for weight loss in the mice. Other such uncoupling compounds have been synthesized chemically. However, this is the first known natural small molecule with uncoupling activity.
Amazingly, when the scientists injected the N-acyl amino acids into obese mice that ate a high-fat diet, the researchers noted significant weight loss after 8 days of treatment. The weight loss was entirely in fatty tissue.
“Here, we identify a secreted enzyme, peptidase M20 domain containing 1 (PM20D1), that is enriched in UCP1+ [uncoupling protein 1-positive] versus UCP1– adipocytes,” the author wrote. “We demonstrate that PM20D1 is a bidirectional enzyme in vitro, catalyzing both the condensation of fatty acids and amino acids to generate N-acyl amino acids and also the reverse hydrolytic reaction. N-acyl amino acids directly bind mitochondria and function as endogenous uncouplers of UCP1-independent respiration.”
The findings from this study were published recently in Cell through an article entitled “The Secreted Enzyme PM20D1 Regulates Lipidated Amino Acid Uncouplers of Mitochondria.”
Brown fat—the color resulting from the large numbers of mitochondria present in the cells—is found in animals as are small amounts in humans, including mixed in with white fat in what are called “beige” cells. Mitochondria are the site of cellular respiration, meaning the conversion of glucose (from food consumed) into the molecule adenosine triphosphate (ATP), which transports chemical energy within the cell to drive its functions.
Brown fat cells are unique in that they consume glucose without making ATP—referred to as uncoupled respiration. Instead of producing ATP, the brown cells use their energy to burn stored calories in fat, releasing significant amounts of heat in the process. It has been postulated that brown fat cells evolved to protect newborns from cold temperatures. Yet, new research suggests that brown fat can be harnessed as a treatment for obesity, and work is underway in a number of labs and companies aimed at increasing brown fat supplies in people—for example by injecting it.
“These data certainly suggest that either PM20D1 or N-acyl amino acids themselves might be used therapeutically for the treatment of obesity and other obesity-associated disorders, such as diabetes and fatty liver disease,” explained senior study author Bruce Spiegelman, Ph.D., director of the Center for Energy Metabolism and Chronic Disease at Dana-Farber, and professor of cell biology and medicine at Harvard Medical School.
Previous work suggested that a mitochondrial protein called UCP1 was the sole source of brown cells' ability to make heat without performing any work. UCP1 is found exclusively in brown and beige fat cells. Yet now, the researchers found an alternate pathway, independent of UCP1, through which the N-acyl amino acids can turn on energy burning in brown cells and also in the liver.
“These data identify an enzymatic node and a family of metabolites that regulate energy homeostasis. This pathway might be useful for treating obesity and associated disorders,” the authors concluded.