The history of mRNA-based medications is now well known. Early research revealed exciting possibilities. At the same time, it posed daunting challenges, such as mRNA’s fragility. The molecule would break down before it could deliver its message to the right part of the body. Eventually, key challenges were addressed by technological advances. For example, carefully prepared mRNA came to be protected by fatty droplets (lipid nanoparticles). Indeed, the first mRNA-based vaccines to incorporate fatty protection were developed against the Ebola virus. However, mRNA didn’t see worldwide commercial application until the COVID-19 pandemic led to the urgent development of vaccines by Moderna and Pfizer-BioNTech.
With billions of COVID-19 vaccines now delivered worldwide, mRNA technology commands interest. To share eagerly awaited news about the next generation of mRNA-based vaccines and therapeutics, the organizers of the RNA Leaders World Congress prepared a comprehensive program for their March 2022 event. The program covered subjects such as advances in delivery mechanisms, techniques for enhancing protein expression, and projects designed to bring mRNA-based and -targeted therapeutics to a wider group of patients. Outstanding presentations from the event are highlighted in this article.
Widening mRNA applications
Thanks to the COVID-19 pandemic, how mRNA vaccines work is widely understood. An mRNA vaccine delivers mRNA into muscle cells. There, the mRNA provides instructions for making the spike protein that is found on the surface of the COVID-19 virus. The protein is displayed on the cell surface, so the protein can trigger the immune system to produce antibodies against it. Subsequently, the immune system retains a memory and can respond quickly if it encounters a SARS-CoV-2 virus using a similar spike protein to enter body cells.
Messenger RNA has other applications, such as restoring the function of damaged tissue. “RNA therapeutics lends itself well to regenerative medicine. You need to inject it only once or twice to start a process to regenerate an organ or tissue,” said Klaas Zuideveld, CEO of Versameb, a company focused on the discovery and development of RNA-based drugs that can modulate protein expression and simultaneously attenuate multiple therapeutic targets.
At the RNA Leaders World Congress, Zuideveld presented on the company’s successful preclinical work using mRNA to treat stress urinary incontinence (SUI). “SUI is a very large indication that affects lots of women,” he pointed out. “Until 10 years ago, the mechanism of the disease wasn’t well understood. And there are no therapeutic options whatsoever—just physical therapy and surgery.”
SUI is caused by damage to the urinary sphincter. This damage, which often accompanies natural childbirth or results from aging, can prevent the urinary sphincter from closing properly. Versameb applies the mRNA directly to the urinary sphincter muscles. According to Zuideveld, the mRNA increases the expression of a naturally occurring protein and triggers the muscle’s regenerative process.
The company uses a modified form of mRNA that is more effective than unmodified mRNA. That is, the modifications allow the regenerative process to be triggered with less mRNA. “We show a 20-fold increase in potency in our preclinical work,” he asserted. “A small amount of mRNA injected into muscles leads to therapeutic levels of protein.” (This observation followed the simulation of natural delivery in rats.)
A single injection of mRNA rebuilt within nine days the urinary sphincter of the rats to its pre-pregnancy functionality. Following this preclinical work, Versameb intends to perform a first-in-human study in patients with SUI. “Investigators and urologists are very excited about being to regenerate this muscle tissue,” Zuideveld stated. “We’re pleased to have [some of] these people as our supporters shaping our clinical program, and they are key to us starting a clinical trial before the end of the year.”
Targeting mRNA with TANGOs
Another company is hoping to use mRNA technology to treat a serious disease caused by the loss of a single functioning copy of a gene. This company is targeting mRNA precursors with antisense oligonucleotides (ASOs), which are short, single-stranded RNAs that are complementary to mRNA. ASO are typically used to inhibit mRNA.
The company is Stoke Therapeutics. It has developed a technology platform—TANGO (Targeted Augmentation of Nuclear Gene Output)—to develop treatments that upregulate protein production. According to the company’s website, TANGO ASOs bind to pre-mRNA, the precursor molecules to mRNA, to help target genes produce more protein.
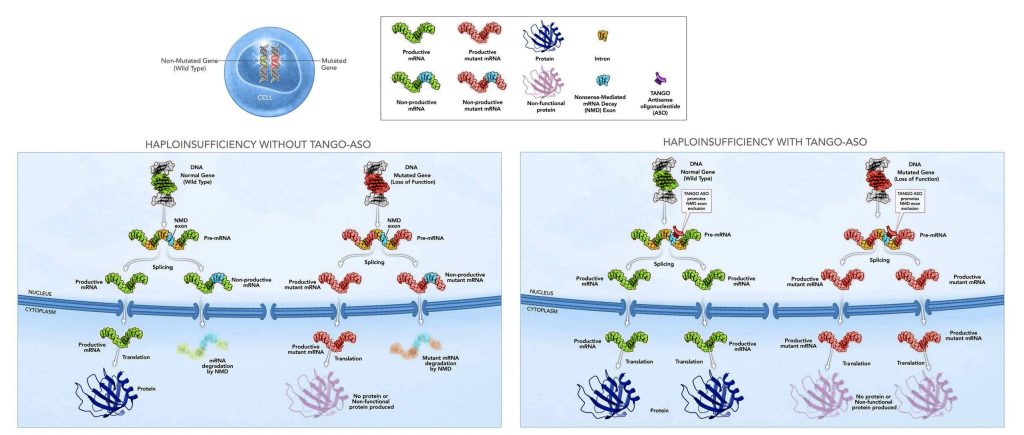
“When we started the company in 2014, it was clear there are many diseases [in which there is one healthy copy of a gene and one mutated copy that fails to produce a protein],” said Huw M. Nash, PhD, chief operating and business officer, Stoke Therapeutics. “We were intrigued by the possibility of increasing expression of RNA to try to compensate for the missing protein.”
At the time, many researchers were looking to use mRNA to synthesize the missing proteins, but this approach was largely unsuccessful because it triggered the immune system, Nash explained, and the applications of mRNA subsequently pivoted to vaccines. “We realized that ASOs could boost protein expression from the wild-type gene back to almost normal levels, and that if ASOs were applied at the right time, they could stop the disease from progressing.”
The TANGO technology is unique, he explained, because most companies have focused on using ASOs to inhibit protein expression in diseases caused by a toxic protein, such as Huntington’s. He claimed that companies that focused on boosting protein expression primarily targeted diseases where both functioning copies of a disease have been lost. In contrast, he emphasized, Stoke Therapeutics is focused on treating diseases caused by haploinsufficiency, the loss of a single functioning copy of a gene, which halves protein production.
Stoke Therapeutics currently has a lead candidate in Phase I/II studies for Dravet syndrome, a rare form of severe, drug-resistant epilepsy. “Although Dravet has antiseizure medicines, this is the first disease-modifying strategy,” Nash asserted. “It can offer something important by replacing what’s missing in these children.” Stoke Therapeutics has a further candidate drug for autosomal dominant optic atrophy. Preclinical toxicology studies to support future clinical trials are underway this year. We hope to complete those studies this year and progress to the clinic sometime next year.
Boosting mRNA performance
With mRNA now regarded as a safe and proven technology, companies are now working to optimize the dosing and formulation. ReCode Therapeutics is a genetic medicines company targeting the underlying mechanisms of genetic lung diseases, such as cystic fibrosis (CF) and primary ciliary dyskinesia (PCD).
The company is aiming to encode a wild-type version of a healthy protein that can be incorporated into the lung. “We’re also broadening beyond mRNA into gene correction of the lung,” said Angele Maki, PhD, senior vice president and head of business development at ReCode. The company, she detailed, is aiming to make small corrections to the CF transmembrane conductance regulator (CFTR) gene to help restore lung function in CF patients.
To advance its program, the company has licensed a lipid nanoparticle formulation technology from the laboratory of Daniel Siegwert, PhD, an associate professor of biochemistry at the University of Texas Southwestern Medical Center.

“Most [lipid nanoparticle] formulations use four components, and [companies] alter the molecules that form each component,” Maki said. “Ours is a five-component system, and the last one can be our choice of lipid.”
By choosing whether to use an ionizable or charged lipid, users of ReCode’s selective organ-targeting (SORT) lipid nanoparticle technology can target the lungs, spleen, or other organs beyond the liver. This differs from traditional lipid nanoparticles, which are taken up by the low-density lipoprotein (LDL) receptor in the liver. “When lipid nanoparticles go into the blood, they look like cholesterol,” she explained, “whereas our SORT lipid nanoparticle is taken up by different receptors on target organs.” The company hopes that its SORT technology can widen the applications of mRNA therapeutics and that, as gene therapy technologies improve, replacing the entire CFTR gene with a corrected copy will be possible.
Replicate Bioscience, meanwhile, has developed a self-replicating RNA (srRNA) platform with the aim of enhancing protein expression at lower doses than conventional mRNA therapeutics. “We think of conventional nonreplicating mRNA as an instruction manual the cell can use,” said Nathaniel Wang, PhD, the CEO of the company. “That instruction manual falls apart after around two days.”
The srRNA delivers a “copying machine” into the cell along with the instruction manual, Wang explained. “It delivers more protein for a longer time, and how much protein is produced is uncoupled from what is delivered to the cell.” He added that this leads to a dramatic reduction in the dose of RNA that’s needed—perhaps 1,000-fold or even 1,000,000-fold compared with a conventional mRNA.
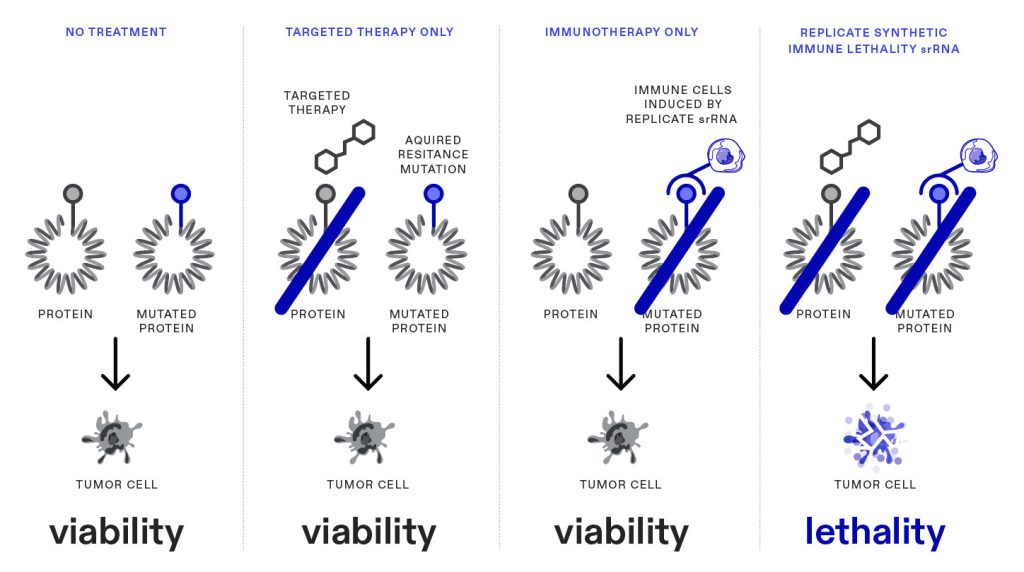
The company, which was founded by srRNA experts, has addressed challenges unique to the manufacture of longer srRNAs. According to Wang, Replicate has succeeded through experimentation and the application of analytics technology, and that the company is confident about taking its technology into oncology and treating inflammatory disease. “We got funding only a year ago, and our pipeline already consists of a half a dozen products and multiple clinical entities,” he asserted.
Among Replicate’s prospective products is an srRNA therapy targeted to the mutations that a tumor is likely to undergo when it is treated with an aromatase inhibitor. “The tumor,” he explained, “can mutate and be destroyed by the immune system, or it can revert to wild type and fall prey to the small-molecule drug.”