Each year, although numerous new pharmaceutical drugs are developed in the lab, most will eventually fail in clinical trials. They are carefully synthesized and exhibit excellent therapeutic efficacy in in vitro and/or in vivo tests. However, one of the main reasons they fail the final tests is the lack of safety and efficacy.
The human body is a delicate machine, and many barriers exist to protect us from pathogens. On the other hand, these same barriers can be the main obstacles for drug delivery. Even if some drugs successfully pass all the clinical trial assessments and eventually are marketed, higher doses are often prescribed, which may induce adverse side effects in the quest for desired therapeutic efficacy.
To enhance drug delivery efficiency, drug carriers that exhibit good water solubility, biocompatibility, nontoxicity, large surface area, small particle size, and some optical properties such as fluorescence are urgently in demand. Under such circumstances, carbon dots (CDs) that possess all the aforementioned properties have drawn an increasing amount of attention as a versatile drug nanocarrier to potentially treat various human diseases.
Overview of carbon dots
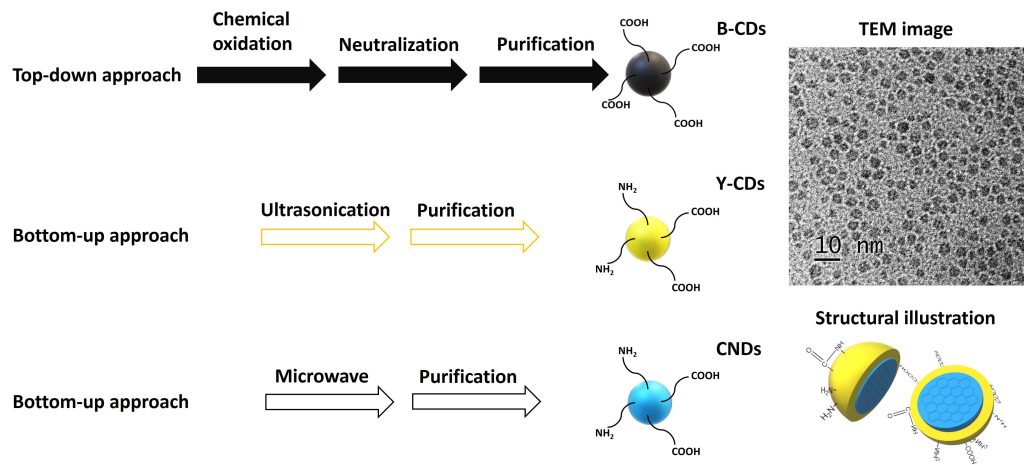
CDs were discovered in the early 21st century. They are defined as carbon-based spherical nanoparticles (NPs; 1–10 nm) composed of graphite-like cores and abundant functional groups as the “shells” that vary with the precursor and synthetic approach.
As a promising drug nanocarrier, CDs own many unique properties that are rarely found in other nanomaterials. For instance, the composition of CDs lacks metals, and CDs can be directly obtained from food and drink, which reveals their nontoxicity, biocompatibility, low cost, and reliable uses in humans. In addition, they are highly soluble in water, which enables them to flow within the human body.
Abundant surface functional groups provide a convenient approach to conjugate CDs with diverse drugs via an EDC/NHS-mediated amine coupling. Furthermore, small particle sizes allow for a high surface-area-to-volume ratio, which benefits drug loading and interactions with the environment.
CDs are also characterized for their excellent photoluminescence (PL) properties and light-harvesting ability, which can be utilized to track their flow and accumulation in human bodies and assist subsequent phototherapies, respectively. With these properties, applications of CDs in nanomedicine are rapidly rising.
Three types of CDs—black CDs (B-CDs), yellow CDs (Y-CDs), and carbon nitride dots (CNDs)—are widely used as drug nanocarriers, and their syntheses are shown in Figure 1. Each demonstrates unique properties. For instance, B-CDs are rich in surface carboxyl groups. Y-CDs display a yellow PL emission, which can help avoid the interference of autofluorescence in in vivo studies. And CNDs exhibit an excitation-dependent PL with an intense emission.
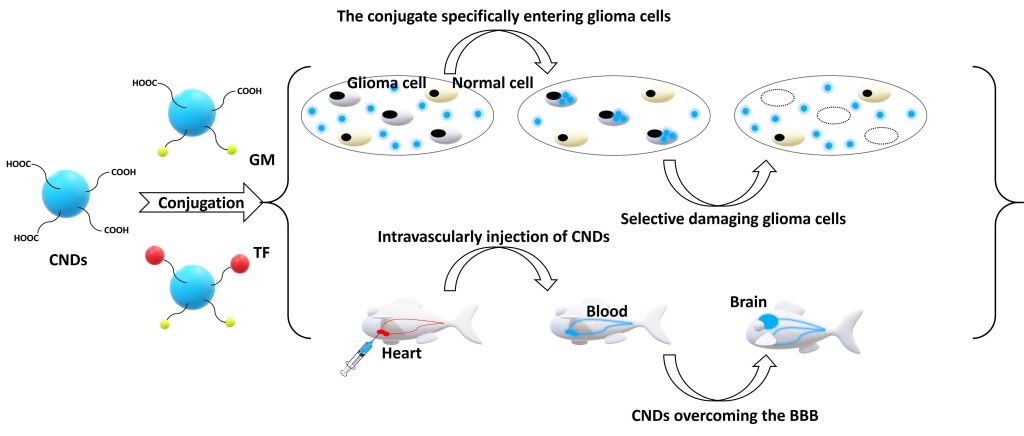
To administer drugs across the blood-brain barrier (BBB), a drug delivery system (DDS) has to be small, low in charge, and low in hydrophilicity. After conjugation with transferrin (TF), B-CDs successfully overcame the BBB. Due to small particle sizes (2–4 nm), low surface charges (zeta potential: from –15 to –25 mV), and amphiphilicity, Y-CDs, CNDs, and their individual conjugates can penetrate the BBB via passive diffusion, confirmed by a zebrafish model study. CDs’ BBB penetration abilities were also generally confirmed with mouse and rat models.
Preclinical CD applications in cancers and neurodegenerative diseases
Glioblastoma is known to be one of the most life-threatening cancers. The major obstacles in the treatment of glioblastoma include the lack of prognosis data and the proper DDS capable of crossing the BBB. Due to these facts, patients can face drastic side effects over a long time span, even after they survive cancer.
To target glioblastoma preclinically, a triple-conjugated DDS was developed with B-CDs, TF (the targeting ligand), and two anticancer drugs (epirubicin and temozolomide), whose average particle size was around 3.5 nm. In vitro studies were performed, and the triple-conjugated system displayed a synergistic effect on the inhibition of glioblastoma cells with a mortality rate of 86% in comparison to single- or dual-conjugated systems.
CNDs were observed to selectively target glioblastoma cells as demonstrated by Figure 2. When gemcitabine (GM) was conjugated to CNDs, over 95% of glioma cells were killed at 1 μM of the conjugate with an almost 100% viability of noncancerous cells. Further conjugation to TF allowed selective targeting and anticancer activity at a 100-fold lower concentration. The conjugates were shown to effectively damage several other brain cancer cell lines that didn’t respond well to any single treatment of GM.
Lymphoma
Diffuse large B-cell lymphoma (DLBCL) occurs in a third of non-Hodgkin lymphoma (NHL) cases in the U.S. However, patients with relapsed or refractory disease following frontline therapies have poor prognoses, and salvage chemoimmunotherapy is typically required followed by bone marrow transplantation.
To reduce the pain patients have to suffer, a CD-based DDS demonstrated enhanced drug delivery targeting the DLBCL. In vitro, linking doxorubicin (Dox) and TF to CNDs was 10–100 times more potent than Dox alone against DLBCL cell lines. Cytotoxicity of the conjugate resulted from nuclear entry of Dox, facilitating the breakdown of double-stranded DNA and cell death. Meanwhile, the new conjugate proved to be safe to administer in vivo, and it improved the overall survival of mice while controlling the patient-derived xenograft tumors.
Alzheimer’s disease
Alzheimer’s disease (AD) is an irreversible neurodegenerative disorder that progressively worsens over time. It slowly destroys a person’s memory and abilities to carry out simple tasks. The Alzheimer’s Association states that it is the sixth leading cause of death in the U.S. currently without a cure. In such a context, CDs that exhibited inhibitory effects on more than one pathogenic aspect of AD, such as amyloid, tau, and inflammation, are promising nanodrugs.
B-CDs inhibited amyloid-beta (Aβ) 42 and 40 fibrillations. Molecular dynamic simulations (Figure 3) showed that the hydrophilic surface of B-CDs benefits their Aβ fibrillation inhibition. B-CDs were also found to be effective in inhibiting beta-secretase 1 activity and delaying the formation of Aβ 42.
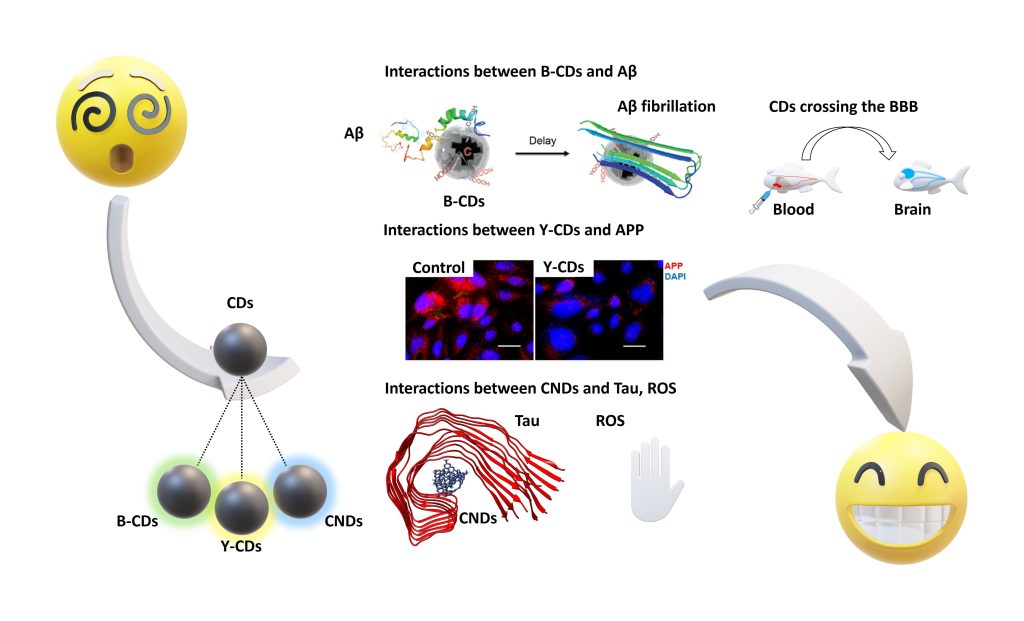
Y-CDs significantly inhibited the expression and secretion of amyloid precursor protein (APP) and Aβ, respectively, after penetrating cell membranes and entering cytosolic compartments (Figure 3). CNDs also inhibited the aggregation of microtubule-associated protein tau (MAPT) by following a dose-dependent pattern. A hydrophobic interaction between MAPT and CNDs was made clear by molecular dynamic simulations (Figure 3). Via a photocatalysis experiment, CNDs were observed to deactivate reactive oxygen species (ROS) and consume the ROS already present in the environment, which can help reduce the oxidative stress on AD patients’ brains.
Once further studies are carried out, CDs will be applied as a versatile drug nanocarrier and/or nanodrug in many more disease preclinical treatments. Possessing properties that enhance therapeutic efficacy, CDs will play a key role in the future treatment of cancers and neurodegenerative diseases.
Yiqun Zhou, PhD ([email protected], [email protected]), is director of R&D at C-Dots (cdots.cc) and C-Dots Nanotec (cdotsnanotec.com).

Comments are closed.