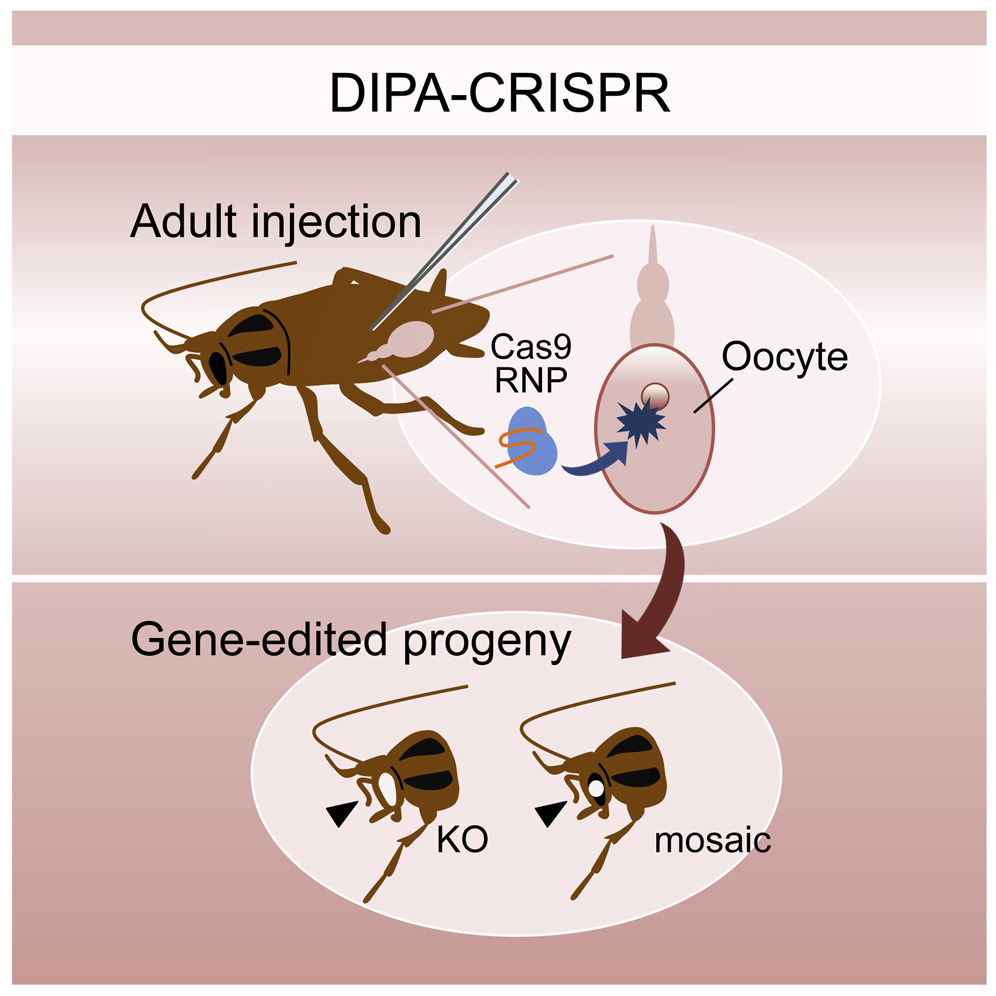
A collaborative study from the University of Kyoto in Japan, and the Institute of Evolutionary Biology in Barcelona, Spain, reported a new method for editing genes in cockroaches and a variety of insect species by directly injecting Cas9 ribonucleoproteins (RNPs) in adult females instead of the challenging endeavor of microinjecting insect embryos, the method currently in vogue. The new method is intuitively named “direct parental” CRISPR (DIPA-CRISPR).
“In a sense, insect researchers have been freed from the annoyance of egg injections,” said Takaaki Daimon, PhD, professor at Kyoto University and senior author of the study. “We can now edit insect genomes more freely and at will. In principle, this method should work for more than 90% of insect species.”
The findings were published in an article titled “DIPA-CRISPR is a simple and accessible method for insect gene editing” in the journal Cell Reports Methods on May 16, 2022.
“By improving the DIPA-CRISPR method and making it even more efficient and versatile, we may enable genome editing in almost all of the more than 1.5 million species of insects, opening up a future in which we can fully utilize the amazing biological functions of insects,” Daimon said. “In principle, it may be also possible that other arthropods could be genome edited using a similar approach. These include agricultural and medical pests such as mites and ticks, and important fishery resources such as shrimp and crabs.”
Historically, it has been very hard to edit insect genes—microinjection into microscopic embryos, unique experimental setups for each insect species, and diverse reproductive systems that preclude access to early embryos, pose just a few of the challenges.
“These problems with conventional methods have plagued researchers who wish to perform genome editing on a wide variety of insect species,” Daimon said.
Daimon’s team injected the editing machinery directly into the main body cavity (haemocoel) of adult female cockroaches and successfully introduced heritable mutations in 22% of hatched eggs. Gene editing efficiency, the fraction of edited eggs among all eggs hatched, varied based on the species. In the red flour beetle (Tribolium castaneum), for example, DIPA-CRISPR achieved an efficiency of more than 50%.
“Commercially available standard Cas9 protein can be directly used for DIPA-CRISPR, which makes this approach highly practical and feasible,” the authors noted. The team demonstrated the inheritance of the edited alleles and the generation of knockin beetles by co-injecting single-stranded oligonucleotides and Cas9 RNPs.
Although the DIPA-CRISPR method is potentially applicable in a variety of insects, it cannot be used in all insects, including the common laboratory model organism, the fruit fly. Moreover, a critical parameter for successful gene editing using DIPA-CRISPR is the developmental stage of the adult females injected. The authors note that in all species tested in the current study, they achieved the highest gene editing frequencies when they injected females undergoing vitellogenesis—the process of yolk deposition in developing egg cells when they actively and non-selectively take up nutrients from their surroundings. These indicate that skill in identifying vitellogenesis in diverse insect species, in addition to a thorough knowledge of insect reproductive anatomies and life histories, is a prerequisite for success in using DIPA-CRISPR.
Although further work is needed in optimizing DIPA-CRISPR, its simplicity and accessibility extends the application of gene editing technology to a variety of common insect models used in the lab as well as pest and pathogens.


