
Vice President, Global Marketing and Strategy, SCIEX
Scans and tests confirmed that I did in fact have lung cancer. I just couldn’t believe it. I was devastated. For me, lung cancer was a death sentence; if you got lung cancer, you died, simple as that, and that’s when I started to think—how am I going to tell my family, my kids?” said Jackie, the subject of a patient story posted by the Roy Castle Lung Cancer Foundation.1 Lung and bronchus cancer is the third most common cancer in the United States, with an estimated 235,760 new cases diagnosed in 2021 and 131,880 deaths—accounting for 12.4% of all new cancer cases and 21.7% of all cancer deaths last year.2 The five-year relative survival rate between 2011 and 2017 was 21.7%.2
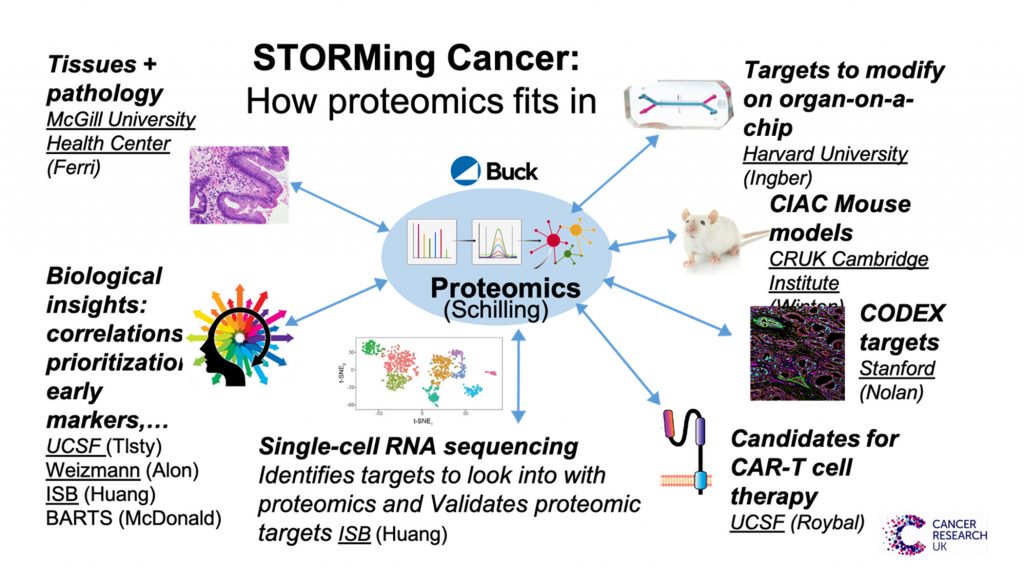
Now, a team of researchers at multiple institutions around the world is taking a novel approach to unravel the mysteries of what causes certain cancers, namely those related to inflammation, such as some lung cancers. This groundbreaking research is being performed as a Cancer Grand Challenges research project, namely, STrOmal ReprograMming Cancer—or STORMing Cancer.3,4 The team, which is led by Thea Tlsty, PhD, a professor of pathology at the University of California, San Francisco (UCSF), is applying a radical new approach to understanding the “nastiest of nasty” cancers. The approach relies on state-of-the-art technologies—many of which are being developed by team members as they go along! Team members are focusing on four types of cancer—esophageal, stomach, colon, and lung—that are associated with chronic inflammation. Cancer cases that have been linked to chronic inflammation account for about 25% of all cancer cases and are estimated to cause 1.7 million deaths worldwide annually.3
Identifying common cancer pathways
Chronic inflammation, such as that resulting from chronic damage to the esophagus by stomach acid in gastroesophageal reflux disease (GERD), can result in abnormalities referred to as metaplasia in the esophageal tissue, recognized as a disease called Barrett’s esophagus or metaplasia.5 In some individuals, metaplasia tissue becomes even more abnormal, progressing to a precancerous state known as dysplasia.5 Dysplasia tissue can then progress to cancer, for instance, in 10–15% of individuals with GERD, who then go on to develop esophageal cancer.5
To understand how chronic inflammation can lead to cancer, STORMing Cancer is examining the building blocks of tissues—specifically, the stromal and epithelial cells, as well as the surrounding extracellular matrix (ECM) and biochemical messengers—and the way they all interact with each other.6
Studies have shown that stromal cells can dictate how adjacent epithelial cells behave. Healthy epithelial cells transform into tumor-like cells when placed next to stromal cells that have been obtained from around a tumor. And vice versa: tumor cells behave like normal healthy epithelial cells when placed next to stromal cells from healthy tissue, despite maintaining an altered genotype.6
These studies indicate that the stroma is dominant and dynamic, as is the ECM by which the stroma mediates its effect on epithelial cells. To elucidate how healthy stromal cells and ECM can reprogram cancers into becoming healthy cells again, the global team is using multiple advanced methods in parallel to analyze biopsy and resection samples from individual patients with cancer and/or the associated chronic inflammatory condition (Figure 1).
Cancer-on-chips for elucidating disease mechanisms
Donald E. Ingber, MD, PhD—a pioneer of organ-on-chip devices7–13 and a co-founder of Emulate14—leads a group at the Wyss Institute at Harvard that is developing organ-on-chip devices that model inflammation-associated cancers. An organ-on-chip device from Emulate is about the size of an AA battery and composed of flexible polymer. It is a microengineered fluidic system that provides human cells with the dynamic environment needed to more faithfully replicate the body in three dimensions (Figure 2).
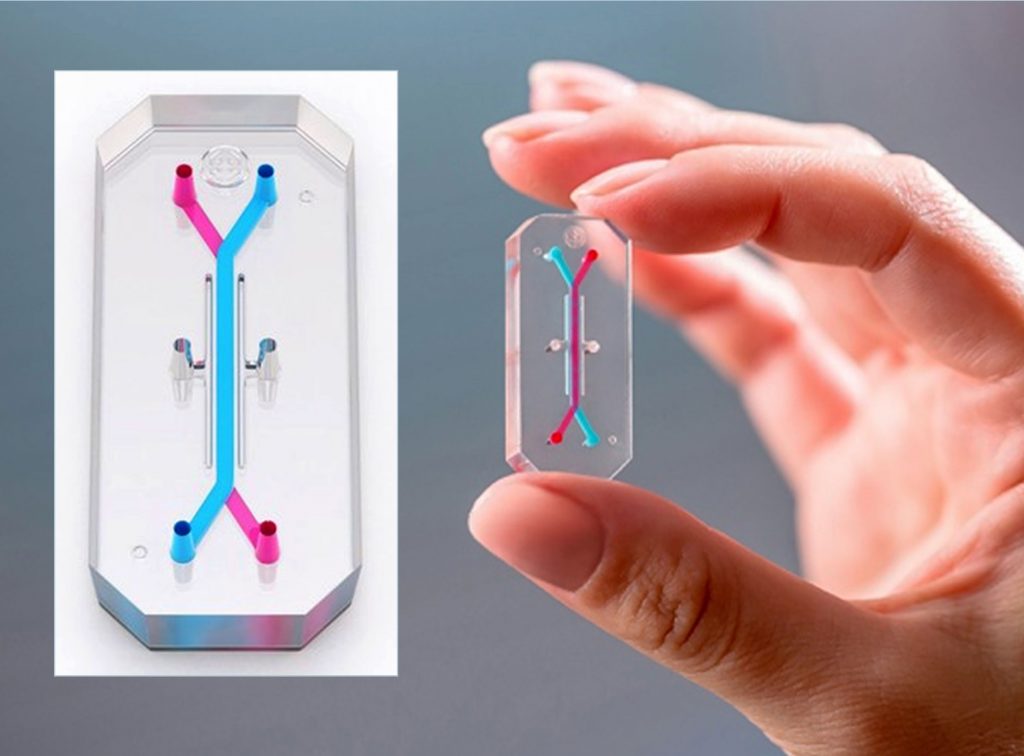
It contains two microchannels that can recreate an extracellular matrix, providing the scaffolding found in the body’s cellular milieu. A flexible porous membrane between the channels allows the co-culture of distinct cell types and the study of phenomena at tissue-to-tissue interfaces. The channels are also designed to recreate the flow of blood and other fluids, providing biochemical surroundings that resemble those in vivo.
Using various analytical approaches, including atomic force microscopy, confocal imaging, cytokine analysis, and transcriptomics, Ingber and his team have identified mechanical and transcriptomic differences between healthy and cancerous cells as well as contributions of the stroma to epithelial cancer progression (personal communication).
These findings correlate with proteomic analyses performed by Birgit Schilling, PhD, and her team at the Buck Institute on pieces of the same patient samples. These analyses revealed robust proteomic signatures indicative of dramatic ECM remodeling between normal and metaplasia stages.6 Some of these changes persisted through dysplasia to full-blown cancer, whereas other changes were transient. (The transient changes occurred only during metaplasia and dysplasia, and then were lost at the tumor stage.6)
The proteomic analyses were also performed using SWATH acquisition, which comprehensively detected and quantified every detectable peptide in the samples. Each analysis produced a multidimensional readout that was so thorough it was essentially a digital archive of the sample that could be reinterrogated later for new information, when no additional patient sample is available.
Thus, as the project progresses and new protein candidates are identified as potential markers for reprogramming precancerous or tumor cells, the team can return to the acquired data to check whether those proteins are detectable and, should they be detectable, whether their abundance differs between samples.6 So far, one of the most important findings is that these proteomic signatures are found across the four cancer types and their associated chronic inflammatory states.6 The next piece of the puzzle is to discover what distinguishes patients with chronic inflammation who go on to develop cancer from those who do not.6
Broadening horizons for translational research and drug development
The use of organ-on-chip platforms is expanding, with many research teams now developing their own models as well as using off-the-shelf options such as those produced by Emulate.15 Organ-on-chip platforms are being used to model tissues such as vascular microvessels, intestinal tissue, and neural tissue, and diseases such as microvascular disease, and Crohn’s disease, and Parkinson’s disease.15–19 Moreover, a bill to modernize the 1938 Federal Food Drug and Cosmetics Act (FFDCA) has been introduced to both houses of Congress.20 The proposed FDA Modernization Act of 2021 aims to amend the mandate for animal testing during preclinical drug development by broadening the scope to accept evaluations of drug safety and efficacy using more advanced and humane technologies instead, where possible.20,21 These technologies include organ-on-chip platforms. Once sufficient validation has been demonstrated, these platforms could pave the way for faster, more effective, and more humane drug development.21
Aaron Hudson, PhD, is vice president and general manager of global marketing and strategy at Sciex, a Danaher operating company.
References
- Roy Castle Lung Cancer Foundation. Jackie Spotted the Difference. November 1, 2021. (accessed January 2022).
- US National Cancer Institute. Cancer Stat Facts: Lung and Bronchus Cancer. (accessed January 2022).
- Cancer Grand Challenges. STORMing Cancer. Cancer Research UK. (accessed November 2021).
- Alvarez J. $26 Million ‘Grand Challenge’ Project Will Probe Role of Inflammation in Cancer. UCSF News. Jan 23, 2019. (accessed November 2021).
- Lowe D, Kudaravalli P, Hsu R. Barrett Metaplasia. Updated 2021 July 26. In: StatPearls. Treasure Island (FL): StatPearls Publishing. 2021 January. (accessed November 2021).
- Hudson A. Memories of Cancer: What the Extracellular Matrix Can Tell Us about Cancer and How to Combat It. Technology Networks. September 15, 2021. (accessed November 2021).
- Maoz BM, Herland A, FitzGerald EA, et al. A linked organ-on-chip model of the human neurovascular unit reveals the metabolic coupling of endothelial and neuronal cells. Nat. Biotechnol. 2018; 36(9): 865–874.
- Herland A, Maoz BM, FitzGerald EA, et al. Proteomic and Metabolomic Characterization of Human Neurovascular Unit Cells in Response to Methamphetamine. Adv. Biosyst. 2020; 4(9): e1900230.
- Benam KH, Novak R, Nawroth J, et al. Matched-Comparative Modeling of Normal and Diseased Human Airway Responses Using a Microengineered Breathing Lung Chip. Cell Syst. 2016; 3(5): 456–466.e4.
- Tovaglieri A, Sontheimer-Phelps A, Geirnaert A, et al. Species-specific enhancement of enterohemorrhagic E. coli pathogenesis mediated by microbiome metabolites. Microbiome 2019; 7(1): 43.
- Si L, Bai H, Oh CY, et al. Self-assembling short immunostimulatory duplex RNAs with broad spectrum antiviral activity. bioRxiv 2021 November 22. DOI: 10.1101/2021.11.19.469183.
- Si L, Bai H, Oh CY, et al. Clinically Relevant Influenza Virus Evolution Reconstituted in a Human Lung Airway-on-a-Chip. Microbiol. Spectr. 2021; 9(2): e0025721.
- Bai H, Si L, Jiang A, et al. Mechanical control of innate immune responses against viral infection revealed in a human Lung Alveolus Chip. bioRxiv 2021 April 27.
- Emulate. About Emulate. Leadership. Donald Ingber. (accessed November 2021).
- Emulate. Products & Services. (accessed January 2022).
- Collins F. NIH Director’s Blog. Making Personalized Blood-Brain Barriers in a Dish. Jun 27, 2019. (accessed November 2021).
- Low LA, Mummery C, Berridge BR, et al. Organs-on-chips: Into the next decade. Nat. Rev. Drug Discov. 2021; 20: 345–361.
- Workman MJ, Gleeson JP, Troisi EJ, et al. Enhanced Utilization of Induced Pluripotent Stem Cell–Derived Human Intestinal Organoids Using Microengineered Chips. Cell. Mol. Gastroenterol. Hepatol. 2018; 5(4): 669–677.e2.
- Junaid A, Schoeman J, Yang W, et al. Metabolic response of blood vessels to TNFα. eLife 2020; 9: e54754.
- Congress.gov. H.R.2565—FDA Modernization Act of 2021. April 15, 2021. (accessed November 2021).
- Emulate. Emulate Endorses the FDA Modernization Act of 2021. October 7, 2021 (accessed November 2021).
Disclaimer
The Sciex clinical diagnostic portfolio is For In Vitro Diagnostic Use. Rx Only. Product(s) not available in all countries. For information on availability, please contact your local sales representative or refer to https://sciex.com/diagnostics. All other products are For Research Use Only. Not for use in Diagnostic Procedures.
Trademarks and/or registered trademarks mentioned herein are the property of AB Sciex Pte. Ltd. or their respective owners in the United States and/or certain other countries.
© 2021 DH Tech. Dev. Pte. Ltd.

