Developers of cancer therapeutics are exploring different possibilities: pushing along existing lines of attack; opening entirely new lines of attack; and coordinating thrusts along multiple lines of attack. At the same time, the developers are interested in limiting collateral damage and fending off counterattacks. That is, they hope to prevent side effects and suppress drug resistance.
If developers are to succeed in maximizing the power of cancer treatments while ensuring patient safety, they will have to rise above the fog of war. Yes, in the war on cancer, as in real war, participants must deal with uncertainty. For example, developers must clarify their therapeutics’ mechanisms of action. Doing so is especially important if novel drug modalities or combination treatments are to be deployed.
The cancer therapeutics highlighted in this article represent refined versions of existing drug types or entirely new kinds of drugs. Although detailed mechanisms of action for some of the therapeutics are still emerging, it is clear that the new drugs are, in general, “smarter”—that is, better targeted. And in several instances, the new drugs demonstrate how development platforms may treat drug components like interchangeable parts. Such platforms may facilitate the rollout of improved bioconjugate therapeutics, cancer vaccines, and cell-based therapies.
Small-format drug conjugates
Cancer drugs that kill cancer cells and spare healthy cells are said to be targeted. Typically, they bind to proteins and receptors that are characteristic of cancer cells, but not healthy cells. Such specificity is demonstrated by the targeted cancer drugs called antibody-drug conjugates (ADCs). They contain an antibody, which binds to a tumor antigen, and a cytotoxic drug, which kills cancer cells. But ADCs have several drawbacks.
“Antibodies didn’t evolve to penetrate solid tumors,” says Nicholas Keen, PhD, chief scientific officer, Bicycle Therapeutics. “They’re much too big to do so effectively.” In addition, ADCs can harm healthy cells even though they are supposed to deliver cytotoxins only to tumors. ADCs can bind to healthy cells that express targeted proteins. ADCs can diffuse from targeted cells to harm innocent bystander cells. And ADCs can release their payloads upon being metabolized, injuring the liver.
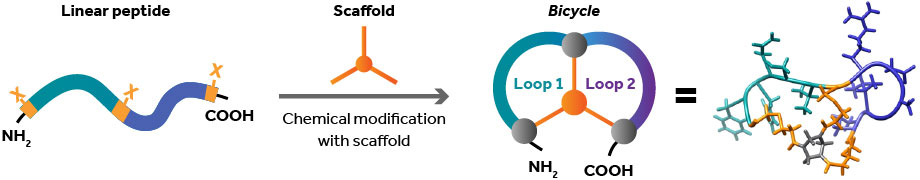
“Because of [these drawbacks], there haven’t been very many successful ADCs,” Keen points out. “But we saw that there was a real opportunity for something that we felt could do the job in a much more engineered way.”
Bicycle Therapeutics develops products that it calls Bicycles. These Bicycles, or bicyclic peptides, are about 100 times smaller than antibodies—small enough to penetrate tumors quickly and effectively. The Bicycles have half-lives of just a few hours. Consequently, Bicycles can be filtered through the kidney instead of the liver, which minimizes systemic exposure.
Each Bicycle therapeutic targets a specific tumor antigen and is armed with a cytotoxic payload. One of Bicycle Therapeutics’ products, BT8009, targets a molecule called Nectin-4 that is overexpressed on certain tumor cells, including bladder, breast, lung, and pancreatic cancers. BT8009 then delivers MMAE, a potent cytotoxin, to those cells.
In preclinical trials, BT8009 outperformed an ADC with more tumor regression and less toxicity, and preliminary Phase I trial data showed that 4 of 11 patients experienced tumor reduction, which ranged from 37 to 89%.
Nanoparticle-based therapeutic vaccines
Immunotherapies stimulate the body’s immune system so that it recognizes and attacks cancer. However, immunotherapies can bring about systemic toxicities. They may be unable to overcome the immune system’s tolerance of cancer. And they may fail to instill immunological memory.
For example, the immunotherapy known as checkpoint inhibition can unleash the immune system by blocking the checkpoint proteins that dampen the immune response. However, checkpoint inhibitors can also cause an immune response against healthy cells and tissues. Overall, checkpoint inhibitors have a success rate of just 15 to 20%.
To address the limitations of existing immunotherapies, PDS Biotechnology has developed a T-cell-activating system called Versamune. It consists of structure-specific cationic lipids (which spontaneously form virus-sized nanoparticles) and antigens (which are custom designed for different types of disease).
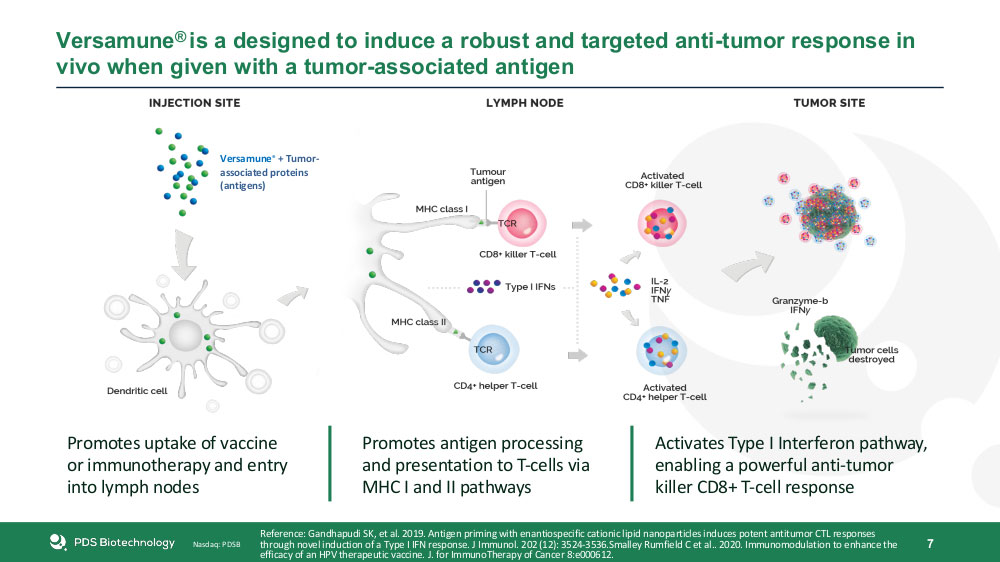
The system is taken up by dendritic cells, which activate and promote the expansion of antigen-specific CD4+ helper and CD8+ killer T cells. The Type I Interferon pathway is also activated, facilitating the recruitment and priming of polyfunctional T cells.
PDS Biotechnology’s lead product, PDS0101, treats a number of human papillomavirus (HPV)-related cancers using a common HPV antigen, HPV16. In Phase I trials, a single dose caused a greater than 20% increase in HPV16-specific killer T cells, leading to the regression of tumors and precancerous cervical lesions. Aside from inflammation at the injection site and lymph nodes, no nonspecific inflammation typical of other immunotherapies was observed.
PDS Biotech has recently begun Phase II trials combining PDS0101 with other common cancer treatments, including checkpoint inhibitors and chemo-radiation. Preliminary results from one trial indicate that in the majority of patients, tumor size decreased more than 30%.
PDS Biotechnology notes that preclinical studies and a Phase I study suggest that Versamune induces immune memory. The company also points to findings indicating that the localized and sustained cytokine induction in the lymph nodes of Versamune has the potential to minimize the risk of systemic toxicity.
Whole-cell therapeutic cancer vaccines
Other companies are trying to develop better immunotherapies. For example, BriaCell Therapeutics is working on genetically engineered whole-cell vaccines. The company’s lead candidate, a targeted immunotherapy for advanced breast cancer called Bria-IMT, is derived from a human breast cancer cell line known for its overexpression of tumor-associated antigens. The cells of this cell line also express granulocyte-macrophage colony-stimulating factor, a protein that promotes the immune response by activating dendritic cells.
The cells are irradiated to ensure replication incompetency. “In developing this technology, we are capitalizing on successful clinical observations in a subset of patients,” says Miguel Lopez-Lago, PhD, the senior director of research and development at BriaCell. Some patients may have had at least one human leukocyte antigen (HLA) match with the version of Bria-IMT that was administered in an early clinical trial.
BriaCell maintains that a genetically engineered whole-cell vaccine can induce a potent immune response against tumor antigens; however, the company acknowledges that such a vaccine may not induce a potent response in tumor microenvironments that shield tumors even if the immune system is activated. To address this issue, BriaCell is combining its therapy with checkpoint inhibitors.
Phase I/IIa trials are still underway, but the company is encouraged by the preliminary data it has collected for the 35 patients that have been treated thus far. Early findings include a good safety profile, an active immune cell response, and some antitumor responses.
“We discovered a strong correlation between the antitumor response that the therapy generates and the repertoire of HLA molecules that the tumor cells express,” Lopez-Lago emphasizes. HLA molecules, which present antigens to the immune system, differ among patients. BriaCell researchers suspect that if Bria-IMT uses HLA molecules different from those naturally occurring in the patient, the patient’s T cells will be unable to recognize the antigen well enough to generate a potent immune response.
BriaCell is improving upon Bria-IMT by engineering 15 unique HLA molecules that will match over 99% of the advanced breast cancer population. This off-the-shelf personalized approach, named Bria-OTS, may provide a more affordable approach to personalized medicine.
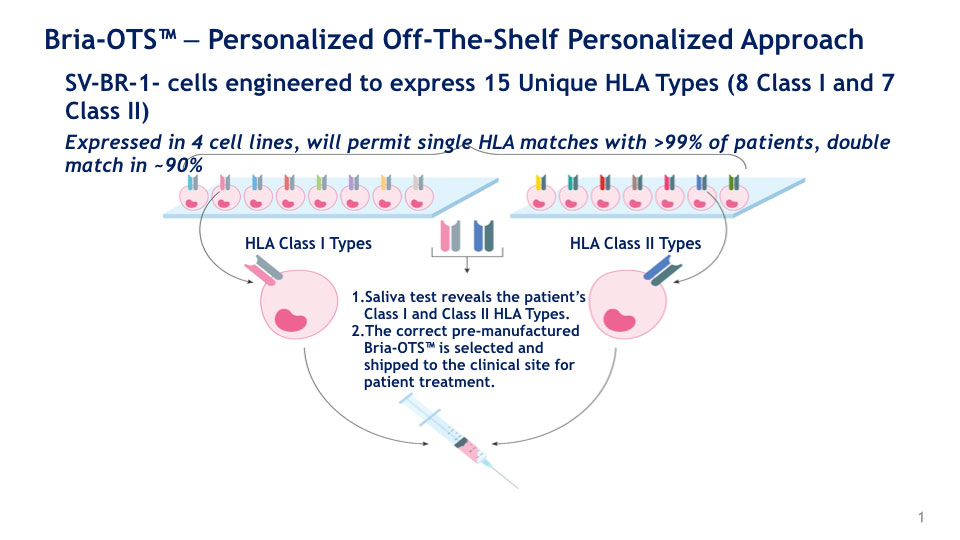
Bria-OTS. The company believes Bria-OTS is most effective when the patient’s HLA type matches the Bria-IMT HLA type. (Fifteen HLA types engineered into Bria-IMT are collectively referred to as Bria-OTS.)
Gene editing and gene repression
While BriaCell develops its own cancer cell line, the contract research organization Horizon Discovery, a PerkinElmer company, provides services and tools, like engineered cell lines, that are designed to help scientists develop new treatments. Horizon’s global senior field application scientist, Catherine Ulich, PhD, notes that the company has amassed vast knowledge and experience in designing gene editing and modulation reagents, as well as in providing technical assistance and resources to customers. She adds that Horizon helps to accelerate development by relieving individual laboratories of some of the burden of generating cellular models.
As a cell engineering company, Horizon specializes in cutting-edge gene editing techniques. One of these technologies is Pin-point, a base editing platform that modifies DNA more precisely and more safely than traditional CRISPR-Cas9 technology. The platform can be used to develop cell therapies, such as CAR T-cell therapies and gene therapies. In addition, this past year, Horizon was the first company to release a synthetic single-guide RNA (sgRNA) for a novel CRISPR interference platform that downregulates gene expression without cutting DNA.
Synthetic single-guide RNA has many advantages over more traditional, expressed guide RNAs. For example, synthetic sgRNA can enable the study of complex phenotypes, the repression of multiple genes at once, and quick initiation of downregulation. In CRISPR interference applications with synthetic sgRNA, gene repression starts within 24 hours after transfection and lasts for up to 96 hours.
According to Ulich, Horizon’s gene repression platform “enables scientists to better understand the biological pathways and processes that have been corrupted in cancer.” These processes can then be targeted for cancer treatment.
Targeting chromatin regulation
One area being targeted for the first time is the chromatin regulatory system. “When the system operates correctly, the right genes are expressed at the right times in the right locations. In the disease state, we have a hijacking of the system, and an aberrant gene expression program runs that drives the disease,” says Steve Bellon, PhD, head of drug discovery, Foghorn Therapeutics. “Our Gene Traffic Control platform has the potential to reverse this aberrant state by tackling the mutations.”
With this platform, Foghorn is able to identify the specific elements in the system that are implicated in disease and a potentially effective drug compound that will address them.
For instance, Foghorn has developed FHD-609, a protein degrader that targets a component of a remodeling complex within the chromatin regulatory system called BRD9. In all synovial sarcomas, a mutation in the system causes cancer cells to rely on this component for survival.
In preclinical studies, FHD-609 has been found to selectively degrade BDR9 and inhibit tumor growth, results that are particularly promising given that synovial sarcoma is often aggressive and has few therapeutic options. A Phase I trial with this therapy began last August, representing the second instance where this new class of therapeutics was administered to a patient. The first was Foghorn’s lead product, FHD-286.
“Both of these represent the first time that people have entered the clinic drugging this new area of biology,” Bellon asserts. “We’re optimistic that we’ll be able to help some of these patients. That possibility is just super exciting.”