Massachusetts Institute of Technology (MIT) engineers, working in collaboration with scientists at Cancer Research UK Manchester Institute, have developed a new way to grow pancreatic “organoids”—tiny replicas of the pancreas—from either healthy or cancerous pancreatic cells. Using a specialized synthetic hydrogel scaffold to mimic the extracellular environment, the researchers were able to grow tiny pancreatic organoid structures that could be used to study the important interactions between pancreatic tumors and their environment. They hope that the new models could help researchers develop and test potential drugs for pancreatic cancer, which is one of the most difficult types of cancer to treat. The researchers showed that the gel could, in addition, be used to grow other types of tissue, including intestinal and endometrial tissue.
Importantly, and unlike some of the animal derived hydrogels commonly used to grow tissue, the synthetic hydrogel scaffold is easy to assemble and can be produced with a consistent composition every time. “The issue of reproducibility is a major one,” said Linda Griffith, PhD, the School of Engineering professor of teaching innovation and a professor of biological engineering and mechanical engineering. “The research community has been looking for ways to do more methodical cultures of these kinds of organoids, and especially to control the microenvironment.”
Griffith and Claus Jorgensen, PhD, a group leader at the Cancer Research UK Manchester Institute, are the senior authors of the team’s paper, published in Nature Materials, which is titled, “A microenvironment-inspired synthetic three-dimensional model for pancreatic ductal adenocarcinoma organoids.“ Lead author is Christopher Below, a former graduate student at the Cancer Research UK Manchester Institute.
Within the body, tumor cells are embedded in what the authors term “a complex environment” that includes a mix of coerced host cells and a “pathologically remodeled” extracellular matrix (ECM), that together supports immune suppression. So, while experimental in vitro models that capture the pathophysiological characteristics of human tumors are “essential for basic and translational cancer biology,” the authors continued, models that faithfully replicate the stromal environment are “… essential to improve the development of therapeutic strategies.”
Traditionally, labs have used commercially available animal-derived gel to grow organoids in a lab dish. However, as the most widely used commercial gel is a complex mixture of proteins, proteoglycans, and growth factors derived from a tumor grown in mice, it is variable from lot to lot, and has undesirable components present, Griffith explained. It also doesn’t always allow for growth of multiple types of cells. “Consequently, interdependencies between tumor cells and the microenvironment are inadequately modeled in this system,” the authors noted.
About 10 years ago, Griffith’s lab started to work on designing a synthetic gel that could be used to grow epithelial cells, which form the sheets that line most organs, along with other supportive cells. The gel they developed is based on polyethylene glycol (PEG), a polymer that is often used for medical applications because it doesn’t interact with living cells. “Contemporary synthetic scaffolds, such as the hydrogel scaffolds based on polyethylene glycol (PEG), offer several advantages to cell- and tissue-derived matrices, including exquisite control over growth conditions,” the researchers continued.
By studying the biochemical and biophysical properties of the ECM, which surrounds organs in the body, the researchers were able to identify features that they could incorporate into their PEG gel to help cells grow in it. “… we set out to develop a rationally designed, synthetic 3D model for pancreatic organoids that simultaneously captures relevant physiological components of the tissue microenvironment,” the team explained. “We envisaged that an ideal model would replicate essential cell–ECM interactions, mimic tissue stiffness ranges observed across normal and tumor-bearing tissues, support co-culture of epithelial and stromal cells, and facilitate growth and development of organoids directly from tissue samples.”
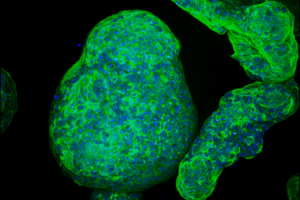
“To enable the growth of normal and cancerous pancreatic organoids from genetically engineered murine models and human patients, essential adhesive cues were empirically defined and replicated in the hydrogel scaffold, revealing a functional role of laminin–integrin α3/α6 signalling in establishment and survival of pancreatic organoids,” the investigators stated. “Altered tissue stiffness—a hallmark of pancreatic cancer—was recapitulated in culture by adjusting the hydrogel properties to engage mechano-sensing pathways and alter organoid growth.”
For their newly reported study, Griffith and Jorgensen wanted to see if the new gel could be used to support the growth of normal pancreatic organoids and pancreatic tumors. Traditionally, it has been difficult to grow pancreatic tissue in a manner that replicates both the cancerous cells and the supporting environment, because once pancreatic tumor cells are removed from the body, they lose their distinctive cancerous traits.
Griffith’s lab developed a protocol to produce the new gel, and then teamed up with Jorgensen’s lab, which studies the biology of pancreatic cancer, to test it. Jorgensen and his students were able to produce the gel and use it to grow pancreatic organoids, using healthy or cancerous pancreatic cells derived from mice. One of the advantages of the new gel is that it is completely synthetic, and can be made easily in a lab by mixing together specific precursors, including PEG and some polypeptides. “We got the protocol from Linda and we got the reagents in, and then it just worked,” Jorgensen said. “I think that speaks volumes of how robust the system is and how easy it is to implement in the lab.”
Other approaches they had tried were too complicated or did not recapitulate the microenvironment seen in living tissues, he said. Using the new gel, Jorgensen’s lab was able to compare the pancreatic organoids to tissues they have studied in living mice, and they found that the tumor organoids expressed many of the same integrins seen in the pancreatic tumors. Furthermore, other types of cells that normally surround tumors, including macrophages, which are a type of immune cell, and fibroblasts, which are a type of supportive cell, were also able to grow in the microenvironment.
The researchers were also able to use their gel to grow organoids from patients’ pancreatic cancer cells, and believe the system could be useful for studying lung, colorectal, and other cancers, and be harnessed to analyze how potential cancer drugs affect tumors and their microenvironment. “This rationally designed and easy-to-use PEG system provides exquisite growth control in a reproducible and defined manner to support future basic and translational studies of human and murine pancreatic organoids within a relevant microenvironment,” they concluded. “This model therefore recapitulates a pathologically remodeled tumor microenvironment for studies of normal and pancreatic cancer cells in vitro.”
Griffith also plans to use the gel to grow and study tissue from patients with endometriosis, a condition that causes the tissue that lines the uterus to grow outside the uterus, and which can lead to pain and sometimes infertility. The researchers have filed a patent on the technology and are in the process of licensing it to a company that could produce the gel commercially.


