Plasmid DNA lies at the heart of viral and mRNA vector production because it provides the coding sequences for gene-based advanced therapy medicinal products (ATMPs). Its manufacture is therefore critical to both the supply and success of these products.
The manufacture of plasmid DNA is certainly critical if viral vectors are produced via transient transfection. This production route calls for the manufacture of up to four plasmids. Otherwise, production may not lead to an initial vector suitable for first-in-human studies.
Production routes that require multiple plasmids put additional pressure on plasmid manufacture, which also needs to supply enough plasmids to sustain growth in the ATMP market. This growth is evident in the number of products in development, as well as in the number of products entering late clinical phase or commercial production. If this growth is to continue, plasmid suppliers will need to modify their service offerings.
The market for plasmid DNA is unusual in that its customers—vector developers—straddle two somewhat conflicting perspectives. That is, these developers see plasmids as commodity products that can be sourced from multiple suppliers. However, the developers also recognize that plasmids pose regulatory challenges because plasmid suppliers maintain their own manufacturing processes and, more critically, their own approaches to quality and testing. Vector developers need to reconcile these perspectives not just during the production and procurement of plasmid DNA, but also through subsequent stages of clinical development.
Plasmid production platforms
Plasmid producers have their own platform processes for the manufacture of plasmid DNA. These processes may be used for multiple plasmid products without being extensively optimized. Although various processes are in use, they all follow the general outline shown in Figure 1.
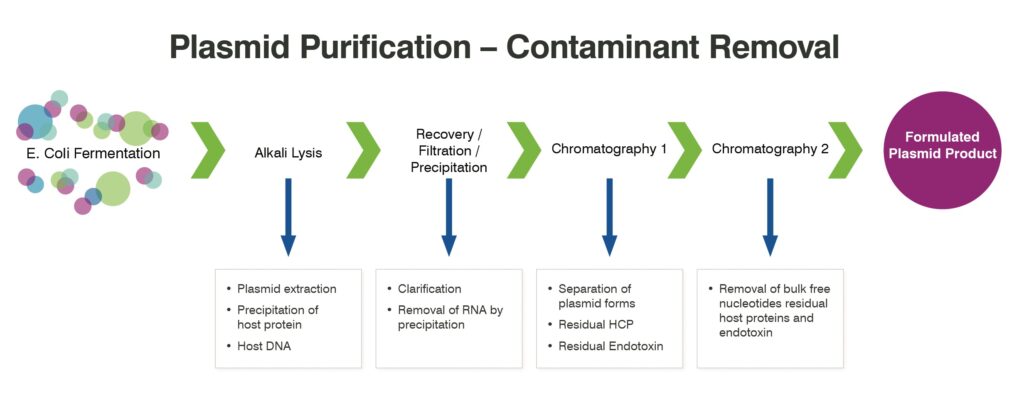
Processes are based on high-density fermentation, usually in a K12-based Escherichia coli strain, the aim being to achieve high specific productivities by constraining growth rates, and to minimize the generation of open and linear plasmid forms during the fermentation. In terms of the downstream processing, the key purification operations are the cell lysis steps (which are based on the Birnboim and Doly process1) and the chromatography steps (which remove key host contaminants and, critically, separate open and closed plasmid forms). A vital element of these processes is their robustness in handling different plasmids, including plasmids of significantly different sizes. (Sizes may range from 2 kb to 16 kb.) These processes also seek to sustain productivity, limiting the need for development process studies and, importantly, keeping timelines short and containing costs.
Traditionally, plasmids were produced in fixed systems. However, with the increasing need to achieve complete product segregation, the drive is toward production in single-use systems. This presents its own challenges around fermentation, access to equipment,
and other key process steps. Although production scales vary from milligram-scale to hundred-gram-scale processes, most systems remain below the 1,000-L scale.
Regulatory status
Another plasmid manufacturing issue is quality assurance. Devising a suitable quality assurance system can be challenging because plasmids are seldom final drug substances. In fact, plasmids may be several steps removed from final drug substances.
When vector production began, there were limited guidelines on the quality of plasmid to be used, and producers took very different approaches. To summarize, in 2005 the European Medicines Agency (EMA) produced guidelines for the production of lentiviral vectors, which required that the plasmid be of a “high quality.” However, the EMA did not define the standards that a plasmid should meet.2 More recently, in 2018, the EMA issued updated guidelines in which plasmid was designated as a starting material. Consequently, it became necessary to sequence and identify plasmids.3 In 2020, the U.S. Food and Drug Administration (FDA) disseminated recommendations regarding chemistry, manufacturing, and control information submitted in investigational new drug applications.4 And this year, the EMA issued more extensive guidance around the grades of plasmid required for the production of a range of ATMPs.5
The guidance provides examples of where Good Manufacturing Practice (GMP) regulations or GMP principles apply in the manufacturing process for ATMPs (Table 1). It also offers suggestions for the use of risk-based approaches around plasmid supply and identifies the need for technical agreements with plasmid suppliers.
Although this document does not propose a requirement for GMP-grade plasmid or Qualified Person (QP) release of plasmid, it does suggest the need for formal technical agreements, placing the onus on vector producers to take a risk-based approach around plasmid supply during clinical development and, subsequently, during commercial supply.
Plasmid service offering
In response to these regulations and guidance documents, plasmid manufacturers have developed service offerings outside of the GMP production platform. These offerings seek to meet the regulatory expectations for the supply of “high quality” plasmids. Plasmid manufacturers have devised a phase-dependent approach that addresses both production scale and quality assurance. The approach is outlined in Figure 2. Here we see that the non-GMP and high-quality (HQ) grades are produced in scales up to 5g, largely to support Phase I/II clinical studies, with most vector producers switching to GMP grade for Phase III and commercial supply. A key element in the aforementioned 2021 EMA guidelines
is the need for the adoption (and justification) of risk-based approaches for plasmid production that are phase appropriate, and the need for quality management systems around vector production operations. Although some may regard the use of GMP-grade plasmid for vector production as being excessive, many are adopting this approach, especially for late-phase production, even when the plasmid is a secondary rather than a primary raw material, as they see this as the best approach of managing the risks around vector production.
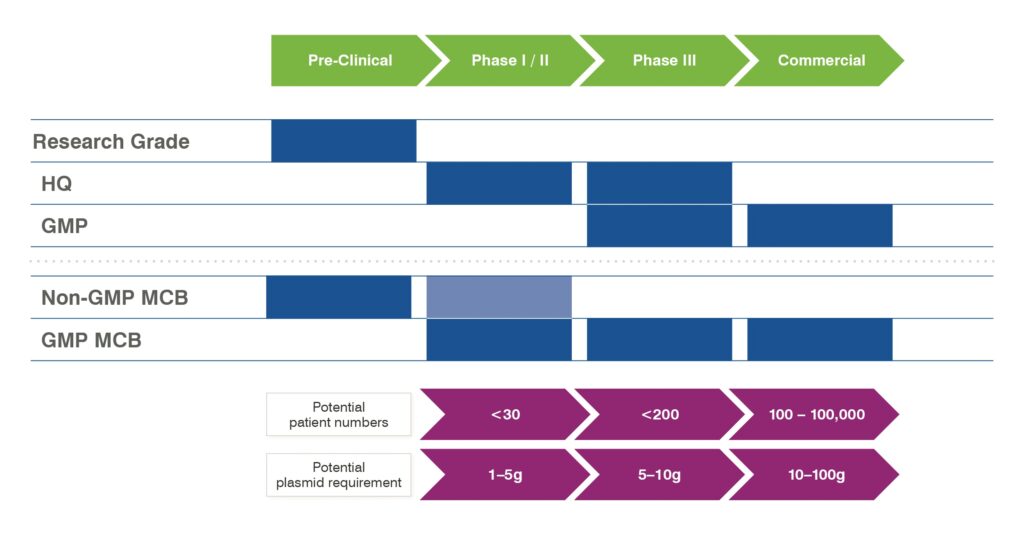
It is also worth noting that most existing large-scale plasmid production facilities are built to operate under GMP and are likely to be maintained as such in the near future; therefore, it is inevitably that large-scale production continues to observe GMP.
In the future, one area that may change is cell bank manufacturing. Both the FDA and EMA documents advocate a move toward the use of master cell banks (MCBs) and working cell banks (WCBs), as well as more stringent testing and characterization of cell banks.
End users will take different approaches to how they achieve the standards they are expected to meet. Plasmid suppliers, however,
must conform to newly clarified standards for plasmid manufacturing. These standards may result in plasmid suppliers adopting comparable quality assurance practices in several
key areas:
• Identity. The plasmids contain coding sequence for the intended product, directly or indirectly, so it is essential the sequence is as intended with no deletions or modifications.
• Cross-contamination. With plasmids being produced in multiproduct facilities, it is essential that there be no cross-contamination of other plasmids produced within the facility. Suppliers that rely on fixed systems will need to develop cleaning procedures and tests to detect plasmids produced previously. Alternatively, these suppliers will need to adopt single-use production platforms.
• Traceability. Clearly, there is a need for knowledge of all the materials going into a process, especially those from
animal origin.
• Documentation. This includes documentation of the manufacturing process (including environmental and training practices), of the materials used within the process, and of the testing and release of products.
Conclusion
The production of plasmid DNA is becoming an increasingly important element in the supply chain for ATMPs. It is therefore going to be ever more critical that plasmid suppliers work with vector producers to ensure that plasmid supplies are maintained while achieving the required quality standards through the development and commercialization of these potentially life-saving medicines. Plasmid suppliers and vector producers can work together effectively if they follow risk-based and phase-dependent
approaches.
Tony Hitchcock is the technical director at Cobra Biologics, a Charles River Company.

