Wounds heal by activating cell division in surrounding tissues that replace lost cells and allow the wound to close. Neural stem cells in mammals have a limited capacity for repair after an injury, and molecular triggers that reactivate their ability to divide are largely unknown.
When neurons attempt to divide, they tend to die. This occurs upon brain injury and in neurodegenerative diseases such as Alzheimer’s Disease, making it important to understand the molecular pathways that coax neurons to divide and eventually target these to prevent undesirable neuronal division.
New research led by scientists at the University of Plymouth, U.K., has uncovered a molecular pathway that sheds light on how cell division is triggered in neurons.
The study, published in the Cell Reports article “Microtubule disruption upon CNS damage triggers mitotic entry via TNF signaling activation,” was funded by the School of Biomedical Sciences and the Peninsula Medical School, University of Plymouth, U.K.
The research is conducted in fruit flies induced to express excessive amounts of a human protein (Tau) associated with Alzheimer’s disease and compares postmortem brain samples from patients who suffered from Alzheimer’s Disease obtained from the South West Dementia Brain Bank (SWDBB).
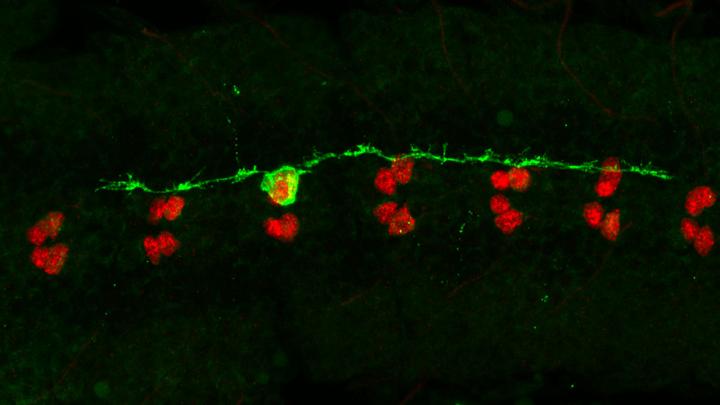
The researchers, Claudia Barros, PhD, associate professor of neuroscience and Torsten Bossing, PhD, Senior Research Fellow at Peninsula Medical School, University of Plymouth, focus on intracellular scaffolds called microtubules in this study that are found in most animal cells including neurons. In Alzheimer’s Disease microtubular networks in neurons can be damaged by the build-up of the protein Tau.
The authors show when microtubules in fruit fly neurons are damaged, neural cell division is triggered through the activation of small signaling kinases, Tak1 (TGF-b activated kinase 1) and Ik2 (IkappaB kinase) that are also activated in the brain in Alzheimer’s disease.
Understanding the dynamics of damaged microtubules and how they trigger cell division in the fly model system, offers a valuable opportunity to find ways to prevent neuronal death following brain injury, or upon neurodegeneration. Abnormal human Tau disrupts the microtubular network in fly brains and in the brains of patients with Alzheimer’s disease. In both flies and humans, Tau can trigger the same signaling cascade as discovered in fly neural cells after microtubule damage.
High levels of Tau accumulation, the scientists report, also correlates with a greater frequency of neurons attempting to divide and a consequent increase in neuronal death. Whether there is a direct causal link between the two remains to be established.
“While other scientists are exploring Tau and how it builds up, we’re looking more at what happens to the cell after it has been damaged,” says Bossing. “The fact that the two identified signaling kinases are found alongside a build-up of Tau in post mortem brains of Alzheimer’s Disease patients suggests that the mechanism identified using fruit flies may act similarly in humans. So, we want to further our studies by using cultured human neurons next. Ultimately, we want to prevent this abnormal cell division entry process from happening in the first place. It’s an exciting piece of work, which we look forward to progressing.”