Humans began culturing animal cells and tissues as early as the late 19th century, when Wilhelm Roux first showed that chick embryos could be cultured in saline solution for a few days. Eventually, human cells were cultured, and techniques were developed to allow in vitro cell culture to recapitulate the body’s cell-level processes outside the body. Two-dimensional (2D) culture has become routine, enabling the discovery and manufacture of life-saving vaccines and therapies. Three-dimensional (3D) cell culture approaches are being developed that attempt to recreate the architecture of the body more realistically, incorporating multiple cell types and tissues along with 3D substrates.
Although in vitro cell culture is of demonstrated utility, animal-based in vivo models are still used at a massive scale within the life sciences industry, for example, in preclinical drug testing. In addition, human clinical trials are still of paramount importance in demonstrating the safety of new therapies and achieving regulatory approvals.
To lessen our reliance on animal models and better guide the use of human clinical trials, scientists continue to develop ever more accurate and realistic in vitro models of the body and its molecular and systemic processes. Sufficiently realistic, responsive, and scalable in vitro models could, to a significant extent, replace in vivo models and clinical testing. They could even advance powerful new multidisciplinary efforts such as multiomics research and precision medicine.
The great news is that an especially promising in vitro modeling approach is already here. It is called organ-on-chip (OoC) technology.
From organ explants to OoCs
Unlike the organ explant culture models that emerged in the 19th century, OoCs are purpose-built microfluidic models that contain living cells and help them sustain their processes. In OoCs, different cell types inhabit preorganized structures that are engineered to resemble those in tissues and to simulate organ functions. Supplied with extracellular matrix materials and other biomaterials, subjected to continuous perfusion and mechanical forces, and monitored via electrical probes, OoCs are quickly filling a gap across the industry.
“With OoCs, we are attempting clinical mimicry in preclinical in vitro models,” says OoC pioneer Donald E. Ingber, MD, PhD, founding director of the Wyss Institute for Biologically Inspired Engineering at Harvard University and professor at the Harvard Schools of Medicine and Engineering. “Moving beyond the limitations of 2D culture, which fails to recapitulate tissues accurately, our organ chips are a pretty transparent window on molecular-scale activities going on within living cells, tissues, or organs.
“Each OoC can be rich with multiomic and real-time monitoring information supporting a plethora of applications. By comparison, a tissue-engineered 3D construct in a bioreactor can be a black box, where real-time visualization and monitoring of individual cell and tissue processes can be limited.”
OoCs on the rise in biotech and biopharma
Josef Atzler, vice president of technology innovation at Molecular Devices, agrees: “Drug candidates fail in clinical studies due to either toxicity or lack of efficacy.” Such defects may be missed in preclinical studies, he continues, “because it’s still common for researchers to develop drugs in the laboratory leveraging animal models or ancient 2D cell lines—which aren’t always as biologically relevant as 3D models. OoC technologies are sophisticated and elaborate, with different cell types that represent tissues and vasculature—environments similar to the human body.”

In our current life sciences landscape, which sees billions invested every year to take promising therapeutics through to market, many candidates ultimately fail. “The statistics show that the industry’s current drug discovery, development, and clinical testing processes are inefficient,” observes Tomasz Kostrzewski, PhD, director of biology at CN Bio Innovations. “It can cost up to $2.6 billion to develop a successful new drug, with each taking up to 10 years to develop.”
Although drugs that reach clinical testing do so only after developers toil for years and spend millions of dollars, just 1 out of 10 of these drugs ultimately secures regulatory approval. Studies suggest that R&D costs could be reduced up to 26%, or almost a third, if developers were to incorporate OoCs into their workflows.
Besides realizing considerable cost savings, OoCs could facilitate precision and/or personalized medicine. To realize this possibility, however, OoC’s will likely need to model human physiology more accurately than the industry’s foundational in vivo models.
“Animal models, used as a proxy for humans in drug discovery and development, cannot consistently and accurately predict human efficacy and toxicity,” Ingber maintains. “Nor can they accommodate personalized medicine, which is already on our doorstep.”
His group has successfully connected multiple OoCs to create one of the first body-on-a-chip platforms. It includes intestine, liver, and kidney chips and has the ability to model how the body processes drugs. In a recent study, Ingber and colleagues used the platform to study the pharmacokinetics of orally and intravenously administered drugs.
The potential for OoCs to facilitate drug development is beginning to be realized commercially by companies such as Emulate, which lists Ingber as a scientific founder. “I remain very optimistic that organ-chip technology will dramatically disrupt pharmaceutical R&D in the coming years,” says Lorna Ewart, PhD, the company’s executive vice president of science. “Animal models are no longer the predictors of human response they once were.”
A similar view is held by Olivier Frey, PhD, vice president of technology and platforms at InSphero. He adds that once OoCs are able to capture the complexity of the human immune system, they will facilitate the evaluation of next-generation biotherapeutics.
“The immune system and its interaction with different tissues under new therapeutic modalities is gaining interest and can be best replicated in multitissue systems,” he explains. “Moreover, the systemic aspects of compound actions can currently be tested only in vivo. Multitissue systems such as OoCs have the potential to move such studies to the in vitro stage for the first time—providing a true alternative to animal testing.”
OoCs in COVID-19 studies and in outer space
OoCs have also played a role in critical COVID-19 research. A “lung airway chip” developed by Ingber’s group in collaboration with Emulate has been used to demonstrate that the antimalarial drug amodiaquine can reduce SARS-CoV-2 infection by about 60%. The drug is currently in COVID-19 clinical trials at multiple sites across Africa.
Besides facilitating the repurposing of drugs to fight a disease that threatens everyone on earth, OoCs have advanced research into conditions that affect much smaller groups of people, such as the select few who venture above the Earth’s atmosphere. For example, Emulate chips have been aboard the International Space Station, where they have been used to study how microgravity influences blood-brain barrier physiology, inflammatory responses, and the ability of the intestinal barrier to ward off bacterial infections.
To enable the study of the intestinal barrier, Emulate provided its Intestine-Chip, which the company plans to continue developing to help researchers better understand the gut microbiome and its role in gastrointestinal disease. Also modeling the gut microbiome with OoC technology is Róisín M. Owens, PhD, professor of chemical engineering and biotechnology, University of Cambridge. She is interested in studying the relationship between the gut microbiome and brain function.
“The interplay between microbes and human cells plays a huge role in human health,” Owen says. “The microbiome can even impact how people process different drugs, for example, cancer therapeutics. [Being in a position to study] fundamental mechanisms and having a playground in which to test various hypotheses, through OoCs, is key to learning how to maintain a healthy microbiome.”
OoCs, organoids, and spheroids
OoC technology is improving in several dimensions. According to Owens, one of these dimensions is the capture of complex biology. Another is the integration of sensor information. Improvement in the latter, she says, is leading to continuous readouts. The collection of sensor information, she details, “can include taking longitudinal samples for downstream analysis and embedding sensors that can measure pH, oxygen, metabolites, and the electrical activity of cells.”
OoCs can also be complemented by other 3D in vitro cell constructs such as organoids. Organoids and OoCs represent orthogonal high-throughput testing systems. Also, organoids can serve as a source of cells for OoCs.
“As 3D self-organized structures containing multiple tissue-specific cell types and at least some of the tissues’ functionality, organoids [constitute] cell systems [that are] very different from those found in OoCs,” Atzler explains. “Organoids provide another great tool for studying developmental biology and understanding disease, alongside OoCs.”
Ingber’s group uses either primary cells or patient-derived organoids as a source of physiologically relevant cells and/or as materials for cultures of particular cell types.
Frey feels that spheroids occupy a crucial stretch along 3D culture’s OoC–organoid–spheroid continuum. He explains, “By combining OoC technology with human-based, multicellular spheroid cultures and connecting different tissue systems, we are getting one big step closer to emulating the complex processes observed in animal models or clinical trials.”
Is scale-up a hold-up?
OoC technology promises to bring powerful new in vitro, in vivo, and clinical testing capabilities to the life sciences industry; however, OoC technology first needs to overcome a number of challenges.
According to Ingber, the ultimate challenge is the bubble. “It kills everything,” he complains. “Having automated systems that can control pressures and flows is therefore important.”
“Cell sourcing can also be a challenge, especially batch-to-batch variability,” he continues. He adds that there is also a requirement for further development of technology for “noninvasive readouts, such as integrated electrodes that provide real-time insights into OoCs in culture.”
The lack of a universal optimal cell culture medium that can work across all cell types in an OoC is another challenge. Ideally, one would be able to grow tissues and organs in a single type of buffer and/or medium without having to rely on additional reagents, many of which are very expensive. “Ultimately,” Owens suggests, “moving to the use of blood or some sort of artificial substitute will be key.”
Frey’s opinion is that the key to future development lies in interoperability of components and systems across manufacturers and companies. “Today, end users are exposed to a high diversity of systems with bits and pieces from different providers,” he notes. “We need more user-friendly, easy-to-use systems.”

Additional challenges are evident in the deliberations between developers and regulatory bodies such as the FDA. “Scale-up and access to quality cells are the largest hurdles we face,” observes Lowry Curley, PhD, CEO, AxoSim. “Customers need the ability to test hundreds of times more drugs than is currently possible.”
Kostrzewski acknowledges that scientists are still unfamiliar OoCs: “Despite the promise of OoCs, it’s difficult to convince scientists to change their strategy from entrenched gold standard 2D cell culture and animal models to an unknown method.” To help broaden the use of OoCs, Kostrzewski and his team at CN Bio are working with the FDA’s Center for Drug Evaluation Research (CDER).
According to a recent announcement, CN Bio and the CDER have expanded a research collaboration to investigate a lung-on-a-chip model for inhaled drug evaluation applications. The collaborators hope to overcome a major challenge to the widespread adoption of OoCs for drug development. This challenge, which was identified by the CDER, is a lack of available quality control and performance criteria that can deliver consistent and reproducible results.
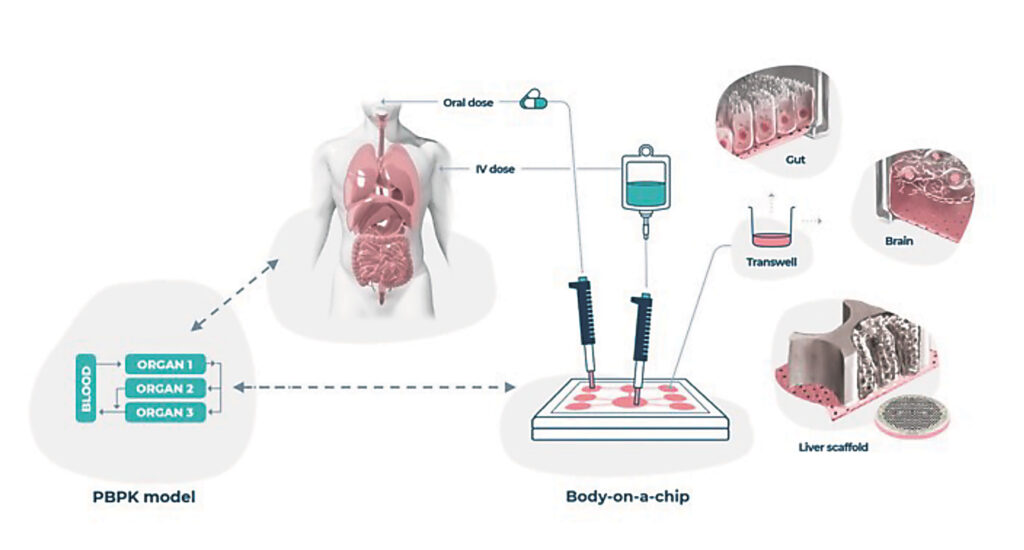
The future of OoCs
In the future, OoC technology may offer “a more human-relevant approach to studying drug candidates,” Ewart suggests. Curley advocates a critical role for OoCs in precision medicine: “Eventually, these technologies will be utilized in precision medicine, predicting if a drug is likely to be successful in clinical trials, as well as whether it will work for a specific patient.” Ingber agrees: “We want patient-specific chips, not a one-size-fits-all approach to future medicines that assumes we are all identical and will all benefit from the same advanced and costly therapies.”
Bringing us firmly out of the “2D cell culture age,” OoCs look set to continue proliferating at a rapid pace, propelling us toward the future of medicine.



