Using a new method called ‘high parameter imaging mass cytometry’ scientists visualize detailed cellular landscapes of the lung in infectious lung diseases at never-before-seen, single-cell resolution.
With this new technique, investigators at Weill Cornell Medicine and New York-Presbyterian target the expression of 36 proteins, to probe into the cellular composition and spatial architecture of human acute lung injury including lung tissue infected by SARS-CoV-2, the coronavirus that causes COVID-19.
The study is reported in the article titled, “The spatial landscape of lung pathology during COVID-19 progression,” published as an accelerated article preview in the journal Nature. The findings provide new insights into the causes of damage in these infectious lung diseases and a detailed resource for future investigations.

“COVID-19 is a complex disease, and we still don’t understand exactly what it does to a lot of organs, but with this study we were able to develop a much clearer understanding of its effects on the lungs,” says Olivier Elemento, PhD, professor of physiology and biophysics, director of the Caryl and Israel Englander Institute for Precision Medicine.
“I think the technological approach we used here is going to become standard for studying such diseases,” says Elemento, who is also associate director of the HRH Prince Alwaleed Bin Talal Bin Abdulaziz Alsaud Institute for Computational Biomedicine at Weill Cornell Medicine and co-Director of the WorldQuant Initiative for Quantitative Prediction, which funded the technology for single cell analysis of tissue.
“The application of technology like what we’ve demonstrated here is going to provide a huge boost to the utility of autopsy-based studies of disease,” Alain Borczuk, PhD, professor of pathology and laboratory medicine at Weill Cornell Medicine and a pathologist at NewYork-Presbyterian/Weill Cornell Medical Center and co-senior author on the study.

“Traditionally for lung, liver, and other organ diseases we have these broad diagnoses that in fact cover multiple distinct diseases—now we have a tool that that will enable us routinely to distinguish among these different diseases, and hopefully make use of those distinctions in treating patients more effectively,” says Robert Schwartz, MD, an associate professor of medicine in the Division of Gastroenterology and Hepatology at Weill Cornell Medicine, pathologist at New York-Presbyterian/Weill Cornell Medical Center and co-senior author on the study. “I think this has the potential to revolutionize medicine.”
Conventional tissue imaging methodologies are limited by the number of proteins they mark simultaneously. Additional challenges include achieving single-cell resolution while retaining spatial or contextual information.
The new technology has significant advantages over fluorescent labelled imaging using confocal microscopy. “Multi-marker confocal imaging uses fluorescent proteins as tags for antibodies as is limited by spectral overlap. In contrast imaging mass cytometry is a high parameter approach that uses metals of different isotopes and is not limited by concerns over signal overlap due to the ability of mass spectrometry to easily differentiate the metals from each other. This allows the use of over 38 different targets simultaneously,” says Robert Schwartz, MD, PhD, assistant professor of medicine at the Sanford I. Weill Medical College of Cornell University and co-corresponding author on the study.

High parameter imaging mass cytometry overcomes these limitations. It uses metal-tagged antibodies that can simultaneously label several dozen molecular markers in tissues. Optimized lasers scan the labeled tissue sections to vaporizing the metal tags, allowing the detection of the distinct signatures of metals that are correlated with the laser position. The technique precisely maps cellular location in the sample, cell surface receptors and other identifying markers.
The authors have validated the technique for paraffin embedded formalin fixed tissues. Although the current study is focused on the lung, it can be deployed for other organs and tissues as well.
The lung being highly porous and vascular has specific handling and imaging challenges. “The lung is highly heterogenous and when injured this is further magnified. As a consequence we developed an imaging pipeline to focus on the different lung structures (e.g. vascular zones, airway zones, and alveolar zones) and collected multiple regions of interest from each patient specimen to fully capture these structural regions as well as the cellular heterogeneity inherent in each patient sample,” says Schwartz.
Using the technique, the authors analyzed over 650,000 cells in 19 lung tissue samples autopsied from patients who had died of severe COVID-19, acute bacterial pneumonia, or bacterial or influenza-related acute respiratory distress syndrome and compared these to four lung tissue samples autopsied from normal individuals with no lung disease.
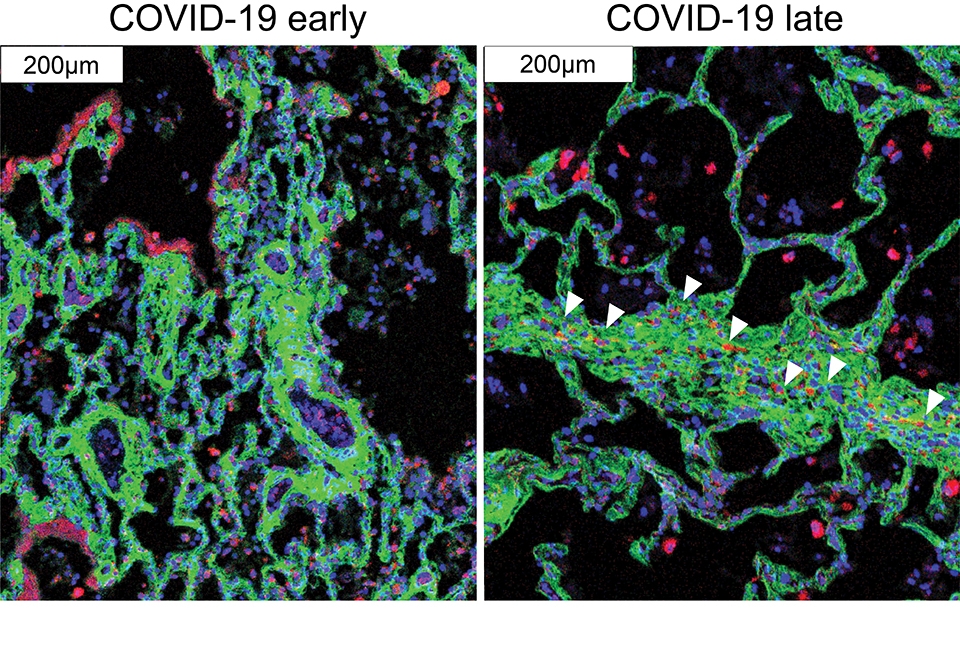
The new technology corroborates what is already known about COVID-19 pathology and provides additional, hitherto unseen, granularity. For instance, the authors show that cells called alveolar epithelial cells that mediate the lungs’ gas-exchange function, are the main targets of infection by SARS-CoV-2. Alveolar epithelial cells are not solely singled out for attack by immune cells that infiltrate into the lungs, which may help explain why inflammation often keeps worsening in severe COVID-19 causing such extensive damage.
The study includes some surprising findings. The authors show age and sex, two major factors in mortality risk for COVID-19, made no apparent difference at the histological level, in severe COVID-19 disease. In addition, white blood cells called macrophages are much more abundant in the lungs of severe COVID-19 patients compared to other lung diseases, while white blood cells called neutrophils are most prevalent in bacterial pneumonia. This distinction may help develop specific treatments for these infectious diseases.

“Similar to other reports, we observed a significant reduction in alveolar lacunar space, increased immune infiltration, and cell death by apoptosis compared with healthy lungs. A high degree of inflammation, infiltration of interstitial macrophages, complement activation and fibrosis is particular to late COVID-19. We find that SARS-CoV-2 Spike positive alveolar epithelial cells do not differentially interact with cells of the immune system despite extensive immune infiltration in the lung. This suggests that COVID-19 may be marked by the lack of “on-target” immunological response, while the high amount of complement activation in COVID-19 lung tissue likely results in indiscriminate “off-target” tissue damage, exacerbating COVID-19 disease and continuing the cycle of inflammation,” says Schwartz.
This suggests that early immunologic interventions particularly targeting indiscriminate complement activation could have therapeutic benefits in COVID-19 treatment.