Immunotherapy for cancer represents a promising alternative for many patients, but so far, it hasn’t delivered long-term benefits to a majority of patients as a standalone therapy. Fortunately, the immune system, with its complex webs of interacting networks and feedback pathways, offers myriad factors that drugs may target to summon tumor-killing resources.
Druggable factors in the tumor microenvironment are now being sought by many developers. When these factors are engaged by therapeutic molecules, it is possible to clear away immune suppressors, to boost the cytotoxicity of immune cells, or to weaken a tumor’s defenses.
Synergizing with checkpoint inhibitors
The receptor tyrosine kinases VEGFR and MET are expressed on many different cell types, and several chemotherapy drugs target them to stop angiogenesis. One of these drugs is cabozantinib, which is a tyrosine kinase inhibitor developed by Exelixis. Cabozantinib was first approved by the FDA in 2016 for patients with advanced renal cell carcinoma because of its ability to inhibit a wide array of tyrosine kinases.
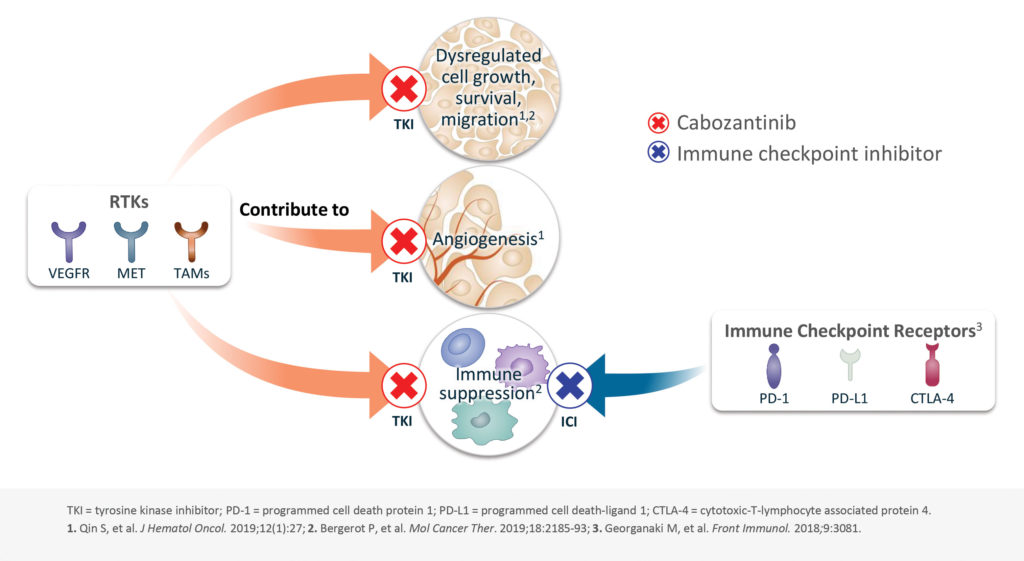
Cabozantinib is the only approved tyrosine kinase inhibitor that targets MET and AXL in addition to the VEGF pathway. According to recent studies, these pathways may be targetable for immune modulation as well. This possibility is being explored by Exelixis, which is combining cabozantinib with immune checkpoint inhibitors (ICIs) to reduce immune suppression and enhance antitumor immunity even in tumor types that have typically been resistant to immunotherapy.
“What was interesting along the way was that we had started to look at the impact of cabozantinib on immune cells,” says Peter Lamb, PhD, CSO of Exelixis. “The results were fairly striking.”
Cabozantinib has a unique immunomodulatory profile, with multiple types of activity. In preclinical studies, the drug was shown to upregulate MHC expression in tumor cells, making them more visible to immune cells. The drug also acts on T cells, both promoting tumor infiltration by CD8+ T cells and suppressing the proliferation of regulatory T cells. Finally, it acts on myeloid cells in the tumor microenvironment, boosting the activity of natural killer cells and reducing the population of myeloid-derived immunosuppressive cells.
“What we’ve come to realize is that the strength of cabozantinib, from a mechanistic standpoint, is the number of things it does,” Lamb notes.
Early data suggests a synergistic effect between cabozantinib and ICIs. “We have a very broad program combining cabozantinib with various ICIs,” Lamb asserts. In particular, Exelixis has partnered with Roche to test cabozantinib plus Roche’s anti-PD-L1 antibody atezolizumab. Phase III trials are ongoing for kidney, lung, and prostate cancer.
“If you take ICIs on their own, prostate cancer has been one of those cancers that’s really not very responsive, but the combination looks significantly better,” Lamb observes. “We think the potential here is very broad and could encompass many different tumor types.”
Targeting fatty acid oxidation
As tumors grow and metastasize, they adjust their metabolism to meet their increased energy demands. Some cancers shift from burning carbohydrates for energy to burning fat. Tempest Therapeutics is pursuing drugs that can stop tumor growth by blocking the fatty acid oxidation pathway.
The transcription factor peroxisome proliferator-activated receptor α (PPARα), which plays a role in regulating the fatty acid oxidation pathway, is overexpressed in several cancers. It has been established that genetically knocking out PPARα halts tumor growth. Following up on this finding, Tempest has developed a PPARα antagonist molecule that is called TPST-1120. This first-in-class molecule is currently in Phase I dose escalation trials, both as a monotherapy and in combination with nivolumab, an anti-PD1 immunotherapy.

cells that rely on fatty acid oxidation.
“We are the only company that’s targeting this pathway with an antagonist,” claims Tom Dubensky, PhD, CEO of Tempest.
Tumor cells aren’t the only cells burning fat for fuel. It turns out that immunosuppressive cells in the tumor microenvironment, including regulatory T cells, M2 macrophages, and tolerogenic dendritic cells also rely on the fatty acid oxidation pathway, and blocking PPARα knocks those cells out of commission as well.
“We’re targeting tumor cells, but also targeting the suppressive side of the immune cell population that resides in the tumor microenvironment,” Dubensky explains. “By inhibiting those two populations, we ultimately inhibit the tumor itself.”
Combining the PPARα inhibitor with anti-PD-1 therapy makes mechanistic sense as well. Fatty acid oxidation drives T-cell exhaustion, and by blocking that metabolic pathway, the drug can rescue exhausted T cells and extend the effectiveness of the ICIs.
“It’s very clear that fatty acid oxidation promotes T-cell exhaustion,” Dubensky points out. “By targeting that along with PD-1, we see real synergy preclinically.”
Certain types of cancer are more susceptible to PPARα inhibition, and Tempest is focusing on hepatocellular carcinoma and cholangiocarcinoma. The mechanism of action of TPST-1120 would synergize with the approved immune checkpoint and antiangiogenic therapies, including bevacizumab and cabozantinib. Early reports from the dose-escalation study have been favorable. “We’ve had a couple of patients who had very long-term stable disease,” Dubensky notes. “We’ve been very encouraged by that sign of clinical activity.”
In addition to evidence of disease modification, the drug also appears to be acting on its target pathway as predicted. “It’s very easy for us to monitor,” Dubensky relates. “We expect to see an increase in the overall level in the blood of triacyl glycerides and long-chain fatty acids, and that’s indeed what we see.”
Targeting glutamine metabolism
Another way to cut off the tumor’s food supply is to block its ability to use the amino acid glutamine for energy and the production of building blocks for amino acids, nucleic acids, and fatty acids. The first-in-class glutaminase inhibitor telaglenastat, from Calithera Biosciences, blocks tumor cell metabolism by preventing tumor cells from using glutamine. Lung cancers that contain mutations that activate the KEAP1/NRF2 pathway are particularly dependent on glutamine, making them sensitive to glutaminase inhibition.
KEAP1/NRF2 mutations make tumors more resistant to oxidative stress, and the tumors become extremely aggressive and difficult to treat. More than 20% of non-small cell lung carcinomas show activation of this pathway. “There’s a very high unmet need,” insists Keith Orford, MD, PhD, chief medical officer for Calithera. “Even getting the best standard-of-care therapy, these patients have a particularly poor prognosis.”
Preclinical data has shown that telaglenastat’s mechanism of action has an added benefit. “There’s a competition within the tumor, between the tumor cells that are trying to eat all the glutamine and the T cells, which need it to activate properly” explains Orford. “If we block the glutamine from being used by the tumor cells, now the immune cells can use it.”
Cell culture experiments have shown that cancer cells deplete glutamine, thereby preventing activation of T cells. Incubation with telaglenastat can block cancer cells from depleting glutamine and reverse the inhibition of T cells. In vivo, inhibition of glutaminase caused an increase in cytotoxic T-cell numbers and may explain the enhanced antitumor activity of anti-PDL1 treatment when animals are co-treated with telaglenastat.
Combining the immune-boosting activity of telaglenastat with its particular activity against KEAP1/NRF2 tumors seemed like an obvious move, Orford recalls. “Putting those together in a front-line setting was a great opportunity. We’re in the right place at the right time.”
The KEAPSAKE trial is a Phase II, randomized, double-blind clinical trial designed to test whether telaglenastat boosts the efficacy of combined anti-PD1 immunotherapy (pembrolizumab) and chemotherapy (carboplatin and pemetrexed) in patients who have non-small cell lung carcinoma with KEAP1/NRF2 mutations. The trial began enrolling in July 2020 at multiple centers. It will include 120 participants and run through 2022.
Using bispecifics to stimulate T-cell activity
If one were to liken the immune system to a car, one could say that suppressor signals, such as PD-1 and PD-L1, correspond to brakes; that certain T-cell activation receptors correspond to ignition switches; and that factors called co-stimulatory receptors correspond to gas pedals. Then, one could discuss ways that one might release the brakes, start the engine, or accelerate cytotoxic activity. Some of us want all the standard equipment.
“The most promising immunotherapies of the past decade, the PD-1 and PD-L1 antibodies, release the ‘brakes’ on T cells,” says John Lin, MD, PhD, head of bispecifics at Regeneron. “The next chapter in immunotherapy, as we see it at Regeneron is that, in addition to these great drugs that address the brakes, we want new ‘keys’ that can turn the T cell on.”
To that end, Regeneron created bispecific antibodies that recognize both a tumor antigen and a receptor on the T cell. One arm of the antibody binds to the tumor antigen, and the other arm binds to the T cell, activating it. The company first made bispecifics against the T cell–activating receptor CD3 but soon realized that the bispecifics aren’t always strong enough on their own to stimulate the T cells. The company decided that a second signal from a co-stimulatory receptor could be helpful.

For its next-generation bispecifics, Regeneron targeted the co-stimulatory receptor CD28. These antibodies bind the tumor-specific antigen with one arm and the CD28 receptor with the other, allowing the tumor cell to prod the T cell into action. “We are basically converting a tumor antigen into a co-stimulatory signal to further activate T cells,” Lin explains.
In animal models, CD28 bispecifics enhanced the antitumor activity of CD3 bispecifics against prostate and ovarian cancers. Importantly, CD28 bispecific antibodies did not trigger a cytokine storm. “It took us several years to figure out how to make it safer so that it would target the tumor and not anything else,” Lin recalls. “We identified a certain type of antibody that would bind to the T cell through the CD28 binding arm but would not activate T cells until the tumor antigen is first detected.”
Regeneron also showed that CD28 increased the efficacy of anti-PD-1 therapy in several different types of cancer. Cancers previously resistant to ICIs became vulnerable when treated with the combination. Further, long-term T-cell memory against the tumors was induced.
Three different CD28 co-stimulatory bispecific antibodies are in clinical trials right now. One is targeting the ovarian cancer antigen MUC16; one is targeting the prostate-specific membrane antigen in prostate cancer; and one is targeting the epidermal growth factor receptor in a variety of solid tumor types. These CD28 co-stimulatory bispecifics are being tested either in combination with anti-PD-1 therapy or CD3 bispecifics.
Next-generation anti-CTLA-4 antibodies
CTLA-4 blockade may have kicked off the immunotherapy revolution, but anti-CTLA-4 monoclonal antibodies benefit only a minority of patients. Even for those who do benefit, the toxicity can be harsh.
In a quest to build a better checkpoint inhibitor, Agenus has created a next-generation antibody that has a higher efficacy and possibly an improved safety profile. The new antibody, AGEN1181, achieved durable responses, including a complete response, in Phase I trials for patients who had already progressed on anti-PD-1 therapy. It was well tolerated by patients across tumor types, with toxicity limited to gastrointestinal and skin effects. AGEN1181 avoids the liver and doesn’t inflict the neuroendocrine toxicity seen with previous anti-CTLA-4 therapies.
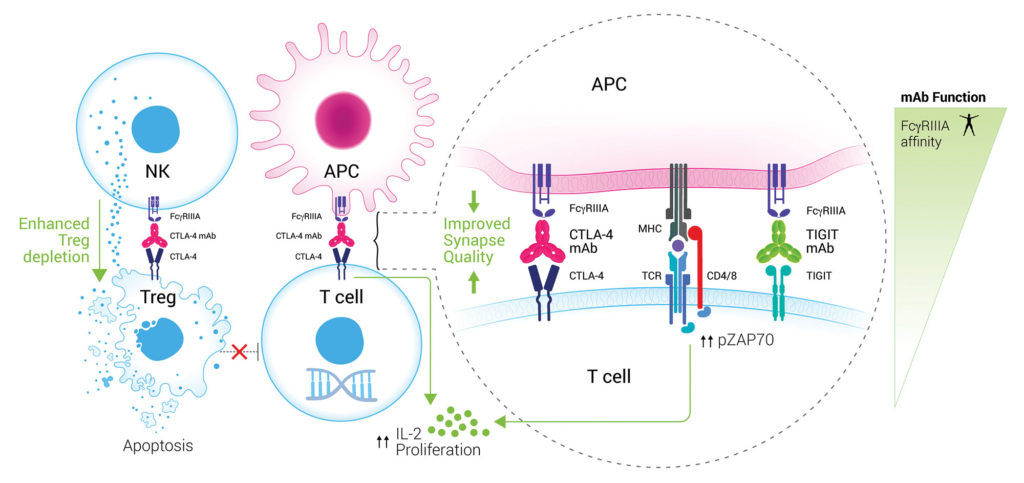
Agenus’ novel approach targets the “back end” of the antibody molecule, the Fc region. In a 2018 paper in Cancer Cell, Agenus reported on the importance of the Fc region in promoting T-cell responses, Treg depletion, and antitumor activity, notes Dhan Chand, PhD, head of drug discovery at Agenus.
Receptors called FcγRs are found on a variety of immune cells, including dendritic cells, macrophages, and natural killer cells. By engineering the Fc region of the antibody, Agenus improved T-cell engagement and boosted crosstalk between FcγR-expressing antigen-presenting cells and T cells. The new antibody has achieved partial and complete responses even in patients traditionally unresponsive to checkpoint inhibitor therapies, including those with low mutational burden or who have low-affinity versions of the FcγRs.
“We believe we have perfected the art of not only directing the Fc to the receptor you want, but also adjusting and fine-tuning the affinity,” Chand asserts. “Through Fc engineering, we were able to broaden the response of these patients, including patients who are refractory to anti-PD1.”
Preclinical data also demonstrated that AGEN1181 reshapes the tumor microenvironment, creating a more favorable ratio of effector T cells to regulatory T cells. This is the first anti-CTLA-4 antibody to demonstrate intratumoral regulatory T-cell depletion in patients with solid tumors.
“That’s a mechanism that’s been chased after but never shown directly clinically,” Chand points out. “We can achieve this because the current molecules are not designed to optimally bind the activating Fc receptors that are needed for antibody-dependent cellular cytotoxicity. We’ve engineered it to enhance the activity and start to leverage biology that we know is important and not captured by the current generation.”