Stem cells hold so much potential for regenerative medicine, it is understandable that so many people should be so impatient to see all that potential realized. But people, the desperately ill among them, need to recognize that stem cells aren’t talismans. In unregulated clinical settings, stem cells can be worthless or even harmful. That’s the bad news. The good news is that stem cells are giving up their profound but decidedly unmagical secrets.
What stem cells lose in mystery, they gain in practicality. They are to be seen as manageable biological units that can, given the right preparation, perform myriad therapeutic applications, less as miracle workers and more as drudges that accept reprogramming and subsequently perform their assigned tasks. They may also sacrifice some of their protean identity, turning into cells that are less stemmy but more effective (and safer) as therapeutic agents. Stemminess may even by bypassed completely, as when cells of one type are directly transdifferentiated into cells of another type.
Even as the preparation of stem cell therapeutics becomes more sophisticated, it is becoming more streamlined, more industrialized. Helping to advance both trends—greater refinement, greater manufacturability—is a new generation of biotech startups. Several of these startups are described in this article. By commercializing the latest stem cell technologies, these startups mean to add to the list of FDA-approved cell-based treatments.
At present, the only stem cell–based therapies on the market are those which use hematopoietic stem cells derived from umbilical cord blood. Yet the FDA has approved cell-based treatments in a range of disorders, including cancer and hematologic disorders. Going forward, cell-based treatments that make use of or are derived from stem cells may proliferate and realize the decades-old dream of countless stem cell cures.
Placenta-derived cells
Celularity, a spinoff from Celgene that was founded in 2017, is developing allogeneic placenta-derived cell therapy products in the areas of hematology, infectious disease, and degenerative disease. Its most advanced programs include placental natural killer (NK) cells for cancer and infectious disease. They are also studying placenta-derived adherent cells in multiple sclerosis and nonhealing wounds in diabetes.
Celularity’s founder and CEO Robert J. Hariri, MD, PhD, says that placenta-derived stem cells can be delivered allogeneically without the need to match the donor to the recipient. Such cells possess immunomodulatory properties that help the mother tolerate the immunologically distinct fetus.
“The technology that resides at Celularity is that the organ is nature’s professional universal-donor material,” asserts Hariri. Moreover, cells isolated from the placenta may contribute to various kinds of therapies, including immunotherapies that depend on chimeric antigen receptor (CAR) T cells or NK cells. Celularity has clinical-stage oncology programs in acute myeloid leukemia, multiple myeloma, and glioblastoma multiforme, as well as clinical-stage infectious disease programs in COVID-19 that use placenta-derived unengineered NK cells.
“The placenta is a very rich source of stem and progenitor cells that can be mass produced at clinical grade in culture expansion in our manufacturing facility,” says Hariri. “And they preserve cell viability and function under cryopreservation, where they have a shelf life that can be measured in decades.” In contrast, an autologous stem cell therapy carries a logistical burden involving collecting the cells from patients, sending them to a facility for processing, and then returning them to the patient after 30–45 days.
Hariri views Celularity’s manufacturing expertise as an important differentiator from other companies in the cell therapy space. He notes that Celularity has recently completed construction on its world headquarters and manufacturing center, which “may be the largest GMP cell manufacturing facility in any independent company.”
Vision restoration
Manufacturing is a consistent challenge for stem cell companies across the industry. Stem cells are difficult to culture and expand, and prone to contamination. That means mastering the technology in the research laboratory is only the first, small step on the path to commercialization. Companies in the space must master batch-to-batch consistency with robust analytics and an independent measurement system to monitor results.
“It’s about the ability to produce the cells in a consistent, quality manner,” stresses Olav Hellebo, CEO of ReNeuron, a company that develops therapeutics from retina-specific stem cells. “That’s the hardest thing. It’s a mix between science and art.”
“There’s no point in having great clinical data and then not being able to reproduce the product that you got the data with,” he continues. “And believe me, it happens all the time.”
ReNeuron has a program for retinitis pigmentosa (RP) that is in Phase I/IIa. Currently, the only available treatment for RP is a gene therapy, Luxterna, marketed by Spark Therapeutics. But Hellebo says that Luxterna works only for patients with RPE65 gene deficiency—a small percentage of patients.
Reneron’s retinal progenitor cell therapy is not designed to replace a gene. “We’re not trying to fix the gene deficiency,” Hellebo remarks. “We are trying to fix the vision, and we are providing trophic support.
“We can see in animal models that our retinal progenitor cells are turning into functional photoreceptors. And that’s pretty magical because you are born with all the photoreceptors you’ll ever have.” In human studies, patients gained about 10 letters in a standard eye chart vision test.
To advance the RP program, ReNeuron had to suspend its promising stroke program. “We have to concentrate our resources where we think we’ll have the biggest return,” Hellebo explains, “and that will be on the retinal program.”
Contaminant-free manufacturing
Glycostem’s approach to the problem of manufacturing its umbilical cord CD34+-derived NK cell product was the development of the world’s first completely closed manufacturing cleanroom. Glycostem’s CEO Troels Jordansen explains, “We take in frozen or fresh umbilical cord blood in a closed bag, and we go through eight steps over 42 days. At the end of that process, the final product, the NK cells, will be available in a bag, and at no time over those eight steps and 42 days will our product have been exposed to the outside environment.”
In a 2017 interview published in BioInformant, Jordansen noted that over 10 years earlier, a technology for stem cell expansion and NK cell differentiation technology originated at the German Cancer Research Center in Heidelberg. Subsequently, this technology was spun out to Radboud University Medical Center, where researcher Jan Spanholtz, PhD, currently Glycostem’s chief science officer, oversaw continued development, which included the building of a closed system manufacturing operation to expand NK cells from umbilical cord blood. This development work culminated in a 10-patient Phase I trial, results from which were published in 2017, in Clinical Cancer Research.
Today, Glycostem is manufacturing a nonmanipulated NK cell product (oNKord) for treating hematological diseases, acute myeloid leukemia, and multiple myeloma, and the company plans to begin patient enrollment in November for a Phase I trial. Glycostem is also working on a range of CAR NK cell products for five different cancer targets, and on NK cells that express T-cell receptors (TCR NK cells).
Jordansen says that based on the studies the company has conducted so far, umbilical cord–derived NK cells made from CD34+ stem cells are superior to NK cells from cord blood, NK cells from peripheral blood, or induced pluripotent NK stem cells. But he cautions that clinical data will be needed for a conclusive comparison.
Stemness without stem cells
Many techniques have been developed over the past 30 years to take advantage of the pluripotency of stem cells by directing them to differentiate into a desired cell type. One of the most surprising of these not only enhances existing stem cell forward reprogramming methods, but also offers the ability to bypass any kind of stem cell or pluripotent state altogether.
A computational system to guide reprogramming started undergoing development in 2011 by scientists affiliated with the University of Bristol. The system was designed to predict the transcription factors needed to achieve transdifferentiation, the direct conversion of cells from one cell type to another. Direct conversion, which avoids taking cells through a pluripotent state, often requires the induction of several transcription factors.
Although direct conversion had been a theoretical possibility, it hadn’t been practical. With over 1,600 known transcription factors and over 200 unique cell types, it would have taken many lifetimes to test enough combinations to find reprogramming “recipes.”
By taking a systematic approach, the new computational system aimed to make direct conversion more efficient and scalable. And it succeeded, as a paper published in Nature Genetics indicated (Rackham et al. 2016; 48: 331–335). When the system was given sufficient data and processing power, it could determine which sets of transcription factors would be needed to produce any target cell type from any source cell type.
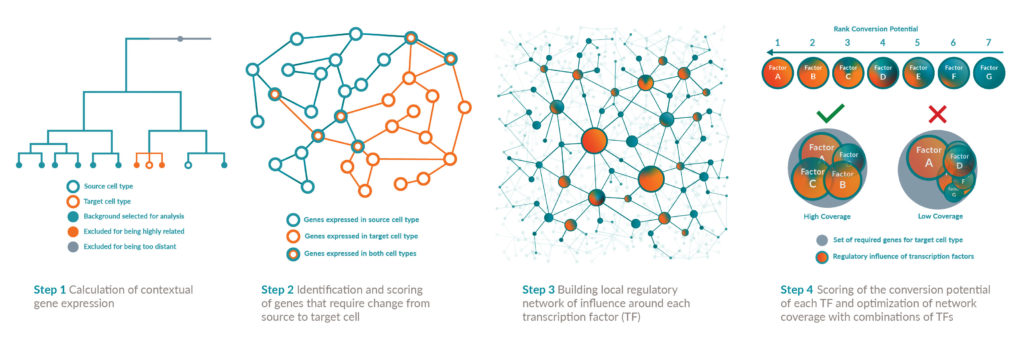
The system, called Mogrify, soon became the basis of a new company, also called Mogrify. Today, the company touts its ability to determine how any human cell type can be converted into any other via transdifferentiation, which involves the transduction of regulatory genes, the modification of DNA expression profiles, and the switching of the cell’s genetic programs.
“The cells lose the features of the starting cell type,” explains Aida Moreno-Moral, PhD, principal bioinformatician at Mogrify. “And they adopt the morphology and function of the desired target cell type.” Mogrify has a pipeline of products in ophthalmology, immunology, and hematology.
“It’s a very exciting time,” declares Moreno-Moral. “Deep cellular profiling has been widely available for less than 10 years. New technologies keep emerging, and the amount of data is increasing exponentially. Mogrify is benefiting from this. With more high-resolution data, we are able to more accurately control cell fate, leading to the development of novel cell therapies.”
Safer, simpler stem cell transplants
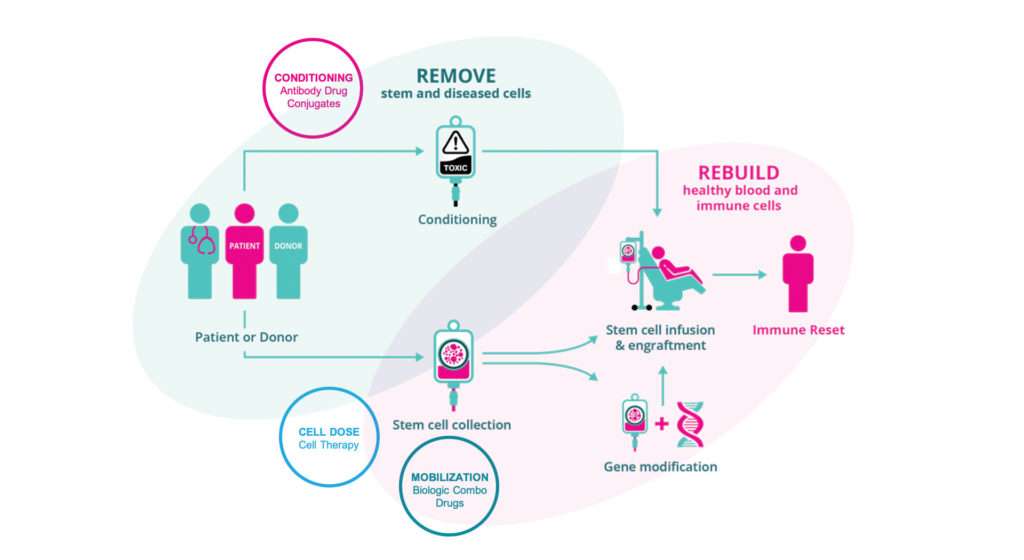
Over a million patients have had a bone marrow transplant. However, today only 40% of eligible patients are able to receive transplants due to obstacles including toxic chemotherapy, difficulty finding a matched donor, and the risk of graft-versus-host disease. That’s according to Jason Gardner, DPhil, CEO and president of Magenta Therapeutics. Magenta, founded in 2016 with technology from Harvard University, is a stem cell biology platform company focused on medicines addressing the obstacles to expanded use of curative stem cell transplants.
Magenta’s lead program, MGTA-145, is a mobilization drug to harvest stem cells for transplant. The current standard of care for mobilizing peripheral blood cells is granulocyte-colony stimulating factor (G-CSF), but it has a number of drawbacks, including a lengthy five days of dosing before cells can be collected. Magenta is studying MGTA-145 in combination with another mobilization therapy, plerixafor, and will soon begin Phase II trials.
That program complements another of Magenta’s pipeline programs, a set of antibody–drug conjugates that target and precisely remove specific disease-causing cells in the body without chemotherapy or radiation in preparation for a stem cell transplant. The most advanced of these antibodies is in preclinical studies for autoimmune disease. Magenta is also working on a cell therapy product for blood cancers and a solution for graft-versus-host disease.
Gardner believes that Magenta’s technology can eliminate the need for chemotherapy. “We don’t need to put the patient through this terrible experience,” he insists. “We have a much better way to collect the best stem cells for transplant. We can use these targeted, very potent medicines from Magenta because we know the stem cell targets and we know how to remove them. This is very exciting, and we believe will be game changing for patients. A successful transplant, when it works, cures the patient.”
A helping hand for stem cells
Cell Guidance Systems is another company building a technology portfolio of therapeutics and reagents that support stem cell therapeutics. The company’s POlyhedrin Delivery System (PODS) packs protein cargo into a protective protein crystal lattice and stabilizes it even at high temperatures. Polyhedrin is an insect virus protein that is very highly expressed in cells and self-assembles into crystals. In the presence of proteases, the PODS crystals slowly break down and release their protein cargo. For stem cell therapy purposes, Cell Guidance Systems is using PODS crystals to deliver growth factors.
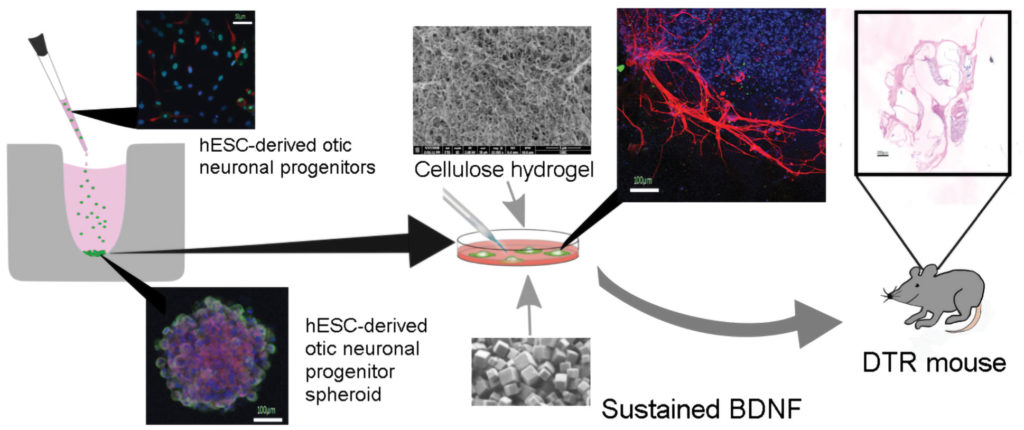
Cell Guidance Systems’ CEO Michael Jones, PhD, says that PODS can make a significant difference in the survival of the transplanted cells. “It’s a little bit like when you move a plant,” he explains. “Often, you’ll put a bit of compost in with the plant, and that helps it thrive. It’s the same thing with cells. You need to give them a little bit of a helping hand when you’re adapting them from one environment into the other.”
The company is working with Akihiro J. Matsuoka, MD, assistant professor of otolaryngology at Northwestern University, to improve the integration of cochlear implants. In this collaboration, PODS containing brain-derived neurotrophic factor (BDNF) are being co-transplanted into the inner ear along with neuronal cells differentiated from pluripotent stem cells. Compared with ordinary growth factors, there is a significantly enhanced quality of neurites of neuronal cells transplanted with the PODS growth factor.
“You can see what a big difference it makes in terms of the quality and functionality of the cell,” says Jones, “if you’re supporting it better by having growth factors available for a long period of time.”

