Human cells and mice cells each express their own version of angiotensin converting enzyme 2 (ACE2), the membrane-bound receptor that serves as a convenient means of ingress for SARS-CoV-2. Because human ACE2 happens to be more welcoming than mouse ACE2, human COVID-19 isn’t easily recapitulated in ordinary mouse models. Genetically engineered mouse models, however, promise to advance studies of COVID-19, whether they aim to detail disease mechanisms or evaluate COVID-19 vaccines and therapeutics.
To ensure that SARS-CoV-2 gets a warm welcome in laboratory mice, scientists representing several research institutions in China have generated a mouse model expressing human ACE2 (hACE2) using CRISPR-Cas9 knock-in technology. The new model captures several features of human COVID-19, including the development of interstitial pneumonia; higher cytokine levels and greater lung damage with increasing age; and gastrointestinal symptoms.
Details appeared May 27 in Cell Host & Microbe, in an article titled, “A mouse model of SARS-CoV-2 infection and pathogenesis.” The article describes how CRISPR-Cas9 knock-in technology was used to generate a mouse model that expresses hACE2.
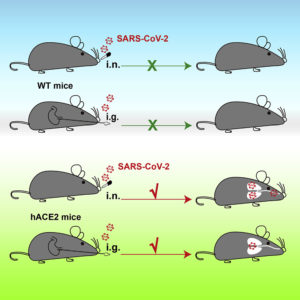
“Compared with wild-type C57BL/6 mice, both young and aged hACE2 mice sustained high viral loads in lung, trachea, and brain upon intranasal infection,” the article’s authors wrote. “Although fatalities were not observed, interstitial pneumonia and elevated cytokines were seen in SARS-CoV-2 infected aged hACE2 mice. Interestingly, intragastric inoculation of SARS-CoV-2 was evidenced to cause productive infection and lead to pulmonary pathological changes in hACE2 mice.”
Wang and his collaborators suggest that their mouse model has several advantages compared with other genetically engineered mice that express hACE2 for modeling SARS-CoV-2 infection. Instead of being randomly inserted, hACE2 is inserted precisely into a specific site on the X chromosome, and it completely replaces the mouse version of the protein.
The scientists also assert that their model is genetically stable, with few differences among individuals. Moreover, the viral RNA loads in the lung are much higher, and the resulting distribution of hACE2 in various tissues better matches that observed in humans.
“The presence of viral RNAs in the brain was somewhat unexpected, as only a few COVID-19 patients have developed neurological symptoms,” noted co-senior study author Cheng-Feng Qin of the Academy of Military Medical Sciences (AMMS) in Beijing.
SARS-CoV-2 S protein, which binds to hACE2 to enter host cells, was also present in the lung tissue and brain cells. Moreover, the researchers identified the major airway cells targeted by SARS-CoV-2 as Clara cells that produce the protein CC10. “Our result provides the first line of evidence showing the major target cells of SARS-CoV-2 in the lung,” maintained co-senior study author Yu-Sen Zhou of AMMS.
In addition, the mice developed interstitial pneumonia, which affects the tissue and space around the air sacs of the lungs, causing the infiltration of inflammatory cells, the thickening of the structure that separates air sacs, and blood vessel damage. Compared with young mice, older mice showed more severe lung damage and increased production of signaling molecules called cytokines. Taken together, these features recapitulate those observed in COVID-19 patients.
When the researchers administered SARS-CoV-2 into the stomach, two of the three mice showed high levels of viral RNA in the trachea and lung. The S protein was also present in lung tissue, which showed signs of inflammation. According to the authors, these findings are consistent with the observation that patients with COVID-19 sometimes experience gastrointestinal symptoms such as diarrhea, abdominal pain, and vomiting. But 10 times the dose of SARS-CoV-2 was required to establish infection through the stomach than through the nose.
Future studies using this mouse model may shed light on how SARS-CoV-2 invades the brain and how the virus survives the gastrointestinal environment and invades the respiratory tract. “The hACE2 mice described in our manuscript provide a small animal model for understanding unexpected clinical manifestations of SARS-CoV-2 infection in humans,” concluded co-senior study author Chang-Fa Fan of NIFDC. “This model will also be valuable for testing vaccines and therapeutics to combat SARS-CoV-2.”


