A Duke University research team has identified a small area of the mouse brain that can profoundly control the animals’ sense of pain. Their studies found a specific subpopulation of nerve cells within the amygdala, which, when activated, unexpectedly turn pain off, rather than on. The amygdala is not a part of the brain that would be an obvious candidate for harboring a pain control center, as it’s generally linked with negative emotions and responses, such as the fight-or-flight response and general anxiety.
The researchers hope that their findings could lead to the development of a new generation of painkillers that specifically target these neurons. “Pain is a complicated brain response,” said Fan Wang, PhD, the Morris N. Broad distinguished professor of neurobiology in the School of Medicine, and senior author of the team’s published paper in Nature Neuroscience. “It involves sensory discrimination, emotion, and autonomic (involuntary nervous system) responses. Treating pain by dampening all of these brain processes in many areas is very difficult to achieve. But activating a key node that naturally sends inhibitory signals to these pain-processing regions would be more robust.”
Wang and colleagues reported their findings in a paper titled, “General anesthetics activate a potent central pain-suppression circuit in the amygdala.”
One of the main effects of general anesthesia (GA) is analgesia, or loss of pain perception, and this can occur independently to loss of consciousness, the authors wrote. Some patients report that even under GA they are aware of conversations in the operating room, but don’t feel any pain, and this suggests that “there could be a specific analgesic pathway that is independent of the loss-of-consciousness effects of GA drugs,” the investigators noted. “People do believe there is a central place to relieve pain, that’s why placebos work,” Wang further commented. “The question is where in the brain is the center that can turn off pain?”
Scientists have contemplated the existence of a central analgesic system since the 1940s. A seminal study by H.K. Beecher, a physician who served in the U.S. Army during World War II, showed that badly wounded soldiers, although alert and not in shock, did not report pain or a need for medication. “This finding triggered the subsequent search for the brain’s internal analgesic system,” the Duke University researchers stated. “Furthermore, the clinically important phenomenon called placebo analgesia also suggests the existence of a central pain suppression system, but the exact circuits carrying out the placebo effect are unknown.”
Wang’s lab has been investigating neurons that are activated, rather than suppressed, by general anesthetics. In a 2019 study, they reported that general anesthesia promotes slow-wave sleep by activating the supraoptic nucleus of the brain. The results from other reported research also indicated that low-dose GA-induced analgesia might act at levels above the spinal cord, leading the team to hypothesize that GA-induced analgesia involves the activation of a specific, central pain-suppressing circuit.
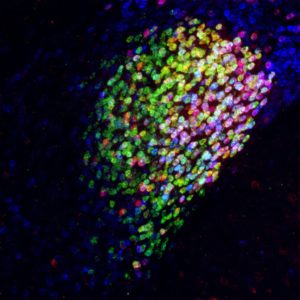
Using technologies that Wang’s lab has pioneered to track the paths of activated neurons in mice, the team found the CeAGA neurons were connected to many different areas of the brain, “… which was a surprise,” Wang acknowledged. By giving mice a mild pain stimulus, the researchers could map all of the pain-activated brain regions. They discovered that at least 16 brain centers known to process the sensory or emotional aspects of pain were receiving inhibitory input from the CeAGA. “Most of the previous studies have focused on which regions are turned ON by pain,” Wang said. “But there are so many regions processing pain, you’d have to turn them all off to stop pain. Whereas this one center can turn off the pain by itself.”
Applying optogenetic techniques that use light to switch on or off specific neurons, the team found that by activating the CeAga neurons they could turn off the self-caring behaviors that a mouse typically exhibits when it feels uncomfortable. Paw-licking or face-wiping behaviors in response to such tests, including temporary pain induction, stopped the moment the light was switched on to activate the anti-pain center. “It’s so drastic,” Wang said. “They just instantaneously stop licking and rubbing.”
Conversely, silencing the CeAGA neurons rendered the mice hypersensitive to mechanical, heat, and cold stimuli. When the scientists dampened the activity of these CeAGA neurons, the mice responded as if a temporary insult that had previously subsided, was again intense or painful. “… when CeAGA neurons were silenced, animals appeared to perceive the subsided injury as becoming intense or painful again,” they wrote.
Encouragingly, activating the CeAGA neurons also suppressed pain-related mouse behaviors in a more chronic neuropathic pain model. The researchers also found that low-dose ketamine, an anesthetic drug that allows sensation but blocks pain, activated the CeAGA center, and that the activity of CeAGA neurons was necessary for ketamine to work. “ … the activity of CeAGA neurons is required for the pain-relieving effect of low-dose ketamine,” they stated.
The researchers suggest that the CeAGA population of neurons may contain different subtypes. “We observed widespread projections of CeAGA neurons to many regions activated by painful stimuli,” they wrote. “It is likely that different subpopulations of CeAGA innervate only a subset of these pain-processing centers, and the entire ensemble of CeAGA neurons is needed to carry out the full analgesic functions.”
And while it’s not known how general anesthetics induce transient or sustained activation of CeAGA neurons, nor what the effects of persistently activating CeAGA neurons might be, the researchers aim to look for drugs that can activate only these cells, as potential future pain killers. “The other thing we’re trying to do is to (transcriptome) sequence the hell out of these cells,” Wang said. This could help them identify a gene for a cell-specific surface receptor that could be developed as a target for highly specific drugs that activate only these neurons, to relieve pain. “Further work aimed at identifying small molecular compounds that can specifically activate these powerful analgesic neurons without the sedative effects of GA drugs could be developed into the next generation of painkillers,” they concluded.


