One day soon, people could carry stickers saying, “These premises are protected by liquid biopsy surveillance.” The premises, of course, are the body’s tissues, which must be protected against malignant processes. Granted, even in the presence of stickers and warning signs, malignant processes persist in their brazen ways. But doing so makes them vulnerable. Tumor cells, for example, cannot help but shed their DNA into the bloodstream, exposing themselves to detection.
Although circulating tumor DNA (ctDNA) is typically scarce and present only in fragments, it is still detectable—provided that the means of detection are sufficiently advanced, that is, capable of enriching targeted sequences, recognizing subtle patterns, and exploiting contextual information.
Some of the most advanced tools for detecting circulating biomarkers were highlighted at a recent industry event that was organized by the Cambridge Healthtech Institute and held in San Francisco. This event—the Fourth Annual Liquid Biopsy Summit—emphasized how the newest tools are bringing liquid biopsies closer to routine clinical application.
Several presenters from the summit contributed their comments to this article. According to these presenters, liquid biopsies are poised to affect every facet of the cancer care continuum, from risk assessment and early detection through monitoring of therapy effectiveness and minimal residual disease detection.
Enhancing error correction
Lucence Diagnostics focuses on cancers common among Asian populations, such as lung, liver, and nasopharyngeal cancer, especially cancers with a virus signature. For example, the cases of nasopharyngeal cancer that occur in Asia account for more than 80% of the cases worldwide. Although the exact cause of most cases of nasopharyngeal cancer is unknown, the disease is closely associated with the Epstein–Barr virus.
In a recent press announcement, Lucent Diagnostics noted that its technology can enable the diagnosis of nasopharyngeal cancer though the detection of cancer-associated viruses such as the Epstein–Barr virus. The company’s tests, which are deployed for clinical use in East Asia, Southeast Asia, and South Asia, can provide physicians and patients with a noninvasive, cost-effective, and fast option for cancer detection and treatment.
In addition to being accredited by the Collampege of American Pathologists (CAP), Lucence Diagnostics expects that it will soon receive a Clinical Laboratory Improvement Amendments (CLIA) certification allowing the company to accept samples from the United States.
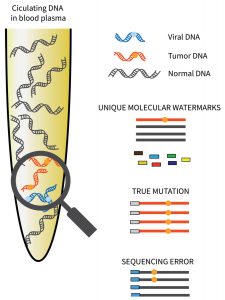
Lucence Diagnostics tests are based on the company’s proprietary amplicon-based ultra-deep sequencing AmpliMARK technology, which detects cancer signals in blood more accurately through amplification. For a cancer patient, this helps with earlier detection and better therapeutic response monitoring.
“A very intelligent error correction is used to amplify a very small signal,” said Jack Challis, PhD, director of healthcare analytics, Lucence Diagnostics. “We are very good at boosting the information from the tumor DNA and reducing the background signal to zoom in to get good characterization of the molecular information in the blood.”
“In one patient, identification of a structural fusion led to a novel opportunity. No FDA-approved drug was available, but the patient was an excellent candidate for two clinical trials.”
“In the future, liquid biopsy will continue to gain prominence as a convenient option and standard of care for patient management, especially for late-stage disease, with a quick turnaround of a week,” Challis continued. “If the liquid biopsy comes back with an actionable opportunity, a patient can start receiving treatment very promptly.”
After completing a molecular characterization, the company generates a report detailing a primary clinical finding, an actionable opportunity, and a set of references and clinical trials, supported by its in-house artificial intelligence (AI)-driven data analytics platform.
Looking at the whole genome
“Liquid biopsy is a good tool to understand the genomics, epigenomics, and proteomics of the tumor over time,” stated Haluk Tezcan, MD, chief medical officer, Lexent Bio. According to Tezcan, Lexent Bio’s liquid biopsy approach involves using a proprietary algorithm to survey the whole genome and discern methylation patterns, which provide a rich source of orthogonal information and exploration of the biology.
“A new enzymatic conversion of unmethylated CpG results in less damage to DNA,” he detailed. This conversion technology, he continued, “allows work with lower DNA input and better conserves whole-genome features of interest.”
Lexent Bio focuses on advanced-stage solid tumors where there is a significant need for a faster response assessment to complement imaging. In later stages of metastatic disease, obtaining useful tissue and characterizing tissue heterogeneity become more difficult.
Unfortunately, for many patients with advanced-stage cancer, a new treatment is likely to fail; current imaging assessments may take 10–16 weeks before indicating whether to consider an alternative approach. During this time, patients may suffer additional side effects, face mounting out-of-pocket costs, and carry an increasing tumor burden, resulting in deteriorated performance status.
Advanced-stage cancers that are progressing in the face of therapy declare their intentions in the peripheral blood within a few weeks of initiating treatment. Given the extraordinarily high prices for newer cancer therapies, it makes sense to invest in both biological metrics for treatment response, as well as traditional imaging studies, as the methods work in tandem.
Two Lexent Bio clinical studies with 92 patients have established that the assay is clinical grade. Additional clinical validation studies will be completed in 2020.
“We believe our whole-genome approach with methylation is the platform of the future,” asserted Tezcan. Emphasizing the point, he declared that Lexent Bio heeds Wayne Greztky’s famous advice: “Skate to where the puck is going to be, not to where it has been.”
“With longitudinal data coupled with the full picture of the entire genome,” Tezcan concluded, “we are set to address the next best option(s) to consider when we identify an ineffective treatment, and with further optimization, the issue of minimal residual disease.”
Upgrading library preparation
The genetic material in liquid biopsies is challenging. Not only is the material low in quantity, it is poor in quality. For example, ctDNA may be fragmented and damaged, containing single strands as short as 20–30 nucleotides. All this material could be efficiently converted into sequenceable molecules by a suitable technology, said Guoliang Fu, PhD, founder, chief scientist, and CEO of GeneFirst.
Such a technology, however, would have address the problem of low sensitivity better than today’s established technologies. Currently, targeted sequencing for detecting ctDNA in liquid biopsies generally uses either ligation-based hybrid capture or amplicon-based enrichment for library preparation.
Ligation-based hybrid capture is technologically mature, and it can be used to detect mutations, fusions, copy number variations, and other structural changes. However, it may involve complicated, multistep experimental procedures. Multiple bead purifications may be needed, many experimental steps may be far below 100%, and recovery rates may be only 5–20% when working with small starting quantities. Adaptations allow easy addition of molecular barcodes for sequencing error correction.
The popular PCR amplicon method is simple, but adding molecular barcodes for error correction can be difficult, and primer pairs may amplify only a fraction of input random fragmented DNA due to break points between primer binding sites. Because of the fixed locations of the pair of primers, the method cannot amplify unknown fusions.

To overcome the limitations of current technologies, GeneFirst developed Adaptor Template Oligo Mediated sequencing (ATOM-seq). According to Fu, ATOM-Seq’s chemistry provides an advanced method for targeting and enriching specific genetic sequences from challenging nucleic acid materials. Such materials may be poor in quality. That is, they may be damaged and highly fragmented, including double- or single-stranded ultra-short sequences (down to 20 bps). Also, the materials may be low in quantity (down to 100 pg).
Using a ligation-independent approach, ATOM-seq adds unique molecular identifiers (UMIs) directly onto the 3′ end of single-stranded starting material with very high efficiency, allowing mutations to be interrogated with ultra-high sensitivity. The method allows for independent targeting of sense and antisense strands of starting material for independent dual-direction coverage, resulting in increased sensitivity and confidence during variant calling. Like ligation chemistries, ATOM-seq amplifies all strands, allowing for detection of unknown fusion events.
The simple protocol generates a final next-generation sequencing (NGS) library in six hours without requiring any hybridization steps. It also has fewer bead purification steps than ligation approaches.
GeneFirst offers a 100-gene pan-cancer panel that detects hotspot mutations. The panel generates 1% allele frequency (AF) detection while using as little as 1–5 ng of DNA, and 0.1% AF detection while using as little as 20–50 ng. Two whole-genome library preparation kits are also available.
Enriching target DNA
Typically, plasma is used to extract cell-free DNA (cfDNA) for liquid biopsy applications. The current standard is solid-phase extraction, such as columns or magnetic particles. Plasma is flushed through the device, genetic material adheres to the surfaces, and then the pH or denvironment is changed to elute the DNA.

traction kit from Phase Scientific Inter-national, enriches for circulating tumor DNA via size selection. The company plans to launch the kit at the end of 2019.
New liquid-phase cfDNA extraction technology for sample preparation is available from Phase Scientific International. The technology, called Phasify, can bridge different applications, including liquid biopsy and point-of-care tests.
“Our technology expands sensitivity significantly by concentrating the target molecules 10–100-fold,” said Ricky Chiu, PhD, the company’s chairman and CEO. “We use two aqueous, biologically friendly phases that allow customization for size selection to get the target to go to a certain level. This gives us better resolution.
“ctDNA is a little shorter, 10–20 bps, than healthy cfDNA. We choose a specific size cutoff to maximize the retention of ctDNA and remove larger DNA pieces, allowing the enrichment of a higher fraction of the usable material than solid-phase extraction methods. The protocol is straightforward—hands-on time 10 is minutes, and overall time is 1 hour.”
The technology is designed to provide highly concentrated samples. The last step precipitates the DNA into a pellet; the pellet is then reconstituted with a buffer of choice at a volume as low as 5 µL. This guarantees that all the genomic material from the sample is available for downstream analysis. Other methods may result in a final volume of 20–80 µL. If only 20 µL of 80 µL is used in the downstream procedure, 75% of the recovered material is lost.
The technology can also be used with urine and saliva biopsies and handles 100–200 mL of sample. Liquid phases are optimized for the various applications.
Phase Scientific International works with diagnostic companies and hospitals to explore and develop new applications enabled by its liquid-phase technology. Research-use-only kits will be launched at the end of 2019 to allow researchers to take advantage of this new sample enrichment tool.
Sourcing tissue and blood
Discovery Life Sciences supplies all types of biosamples for research applications. Recent mergers and acquisitions have increased Discovery’s sourcing and service capabilities, resulting in a suite of solutions tailored to the liquid-biopsy industry.

The first solution is the largest collection of hyper-annotated, matched sample sets that include a formalin-fixed paraffin-embedded resected tumor block with 4 mL of double-spun plasma and the buffy coat from a single patient. Many of these samples come with direct links to clinical information and comprehensive mutational analysis.
“Our prospective oncology network is really powerful,” asserts Rod Beck, Discovery’s vice president of laboratory and scientific services. “Visibility into the electronic medical records at our Discovery Partner sites allows us to match patients directly with our clients’ needs for different types of diseased cohorts.
“On-site study coordinators approach patients who match sponsored studies, ‘consent’ the patients, and collect samples as part of their standard treatment visits. Samples can be shipped directly or be processed locally under study-specific protocols.”
The next part of the integrated liquid-biopsy solution is scientific services, which include cell-based, tissue-based, and genomic provisions. The company’s expert laboratories, pincluding the HudsonAlpha Discovery genomic laboratory, routinely perform testing, including U.S. board-certified pathology analyses, flow cytometry panels, focused or targeted genomic panels, and single-cell sequencing services.
“We are really excited about our ability to be a strong partner by offering the combination of access to patient samples and complex scientific analyses,” Beck declares. “This unique ability to provide both biospecimens and laboratory services at industry scale creates a single chain of custody that speeds liquid-biopsy research timelines, complies with applicable regulatory standards, and provides a single partner from biospecimen acquisition to research, pharmacology, or clinical data delivery.”


