Ten-eleven translocation (TET) proteins have long been known to regulate fundamental biological processes and play important roles in embryonic, neuronal, and hematopoietic development. Through their epigenetic regulation of gene expression, they have been largely implicated as cancer suppressive proteins. Now, a team at La Jolla Institute for Immunology has uncovered how the TET2 and TET3 enzymes contribute to the regulation of immunoglobulin class switching and somatic hypermutation during B-cell activation.
The researchers, led by Anjana Rao, PhD, professor, division of signaling and gene expression, La Jolla Institute, reported that mutations in TET2 and TET3 in mouse B cells diminish the generation of functional IgG antibodies, decreasing the effectiveness of immune responses.
The paper titled, “TET enzymes augment activation-induced deaminase (AID) expression via 5-hydroxymethylcytosine modifications at the Aicda superenhancer” is published in the journal Science Immunology.
TET enzymes are dioxygenases that promote DNA demethylation by oxidizing the methyl group of 5-methylcytosine to 5-hydroxymethylcytosine (5hmC). In this study, the researchers investigated the role of TET proteins during mouse B cell activation by mapping 5hmC distribution across the genome and integrating the data with previous studies of transcriptional and epigenetic changes during B cell activation. In addition, they identify a B cell gene that becomes silenced in the absence of TET2 and TET3, highlighting how critical “epigenetic” control of gene expression is for healthy immune cell function and hinting at why TET loss promotes oncogenesis.
“Previously, people knew that TET proteins were involved in suppressing cancer,” said study-co-author Chan-Wang Jerry Lio, PhD, an instructor at the La Jolla Institure. “But it was difficult to tease out what the normal function of TET genes was because mice developed cancer so rapidly when we deleted them.”
Initiation of immunoglobulin class switching and somatic hypermutation during B-cell activation requires tightly regulated expression of the activation-induced cytidine deaminase (Aicda) gene. This work investigated how the TET2 and TET3 enzymes contribute to the regulation of Aicda expression by studying B cells from mice in which the two enzymes can be inducibly deleted.
The new study circumvents this issue by using an alternate “conditional knock-out” strategy in which experimenters deleted TET2 and TET3 in mature B cells at a time point of their choice in experimental mice. Five days later, they removed the mouse’s B cells and compared their activity with B cells derived from normal mice in which TET2 and TET3 remained intact.
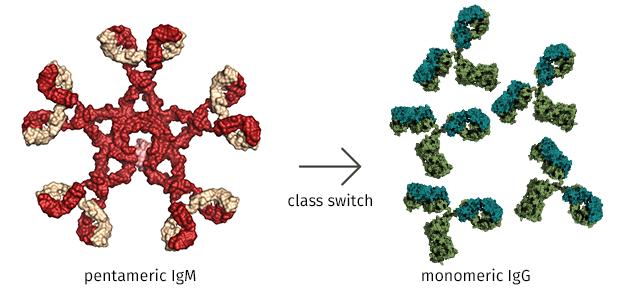
A critical difference was that when stimulated by an experimental pathogen, mutant B cells that lacked TET2 and TET3 produced an overabundance of a class of antibody called IgM, whereas normal control B cells churned out more effective antibodies classified as IgG or “gamma globulin”.
“Antibodies come in different ‘flavors’,” explained Vipul Shukla, PhD, a postdoctoral fellow in Rao’s lab and a co-first author on the study. “The standard flavor (IgM) does a poor job of activating other immune cells. So once a normal B cell encounters a pathogen, it tries to convert IgM antibodies to a more beneficial flavor to mount an effective immune response.”
That “beneficial” flavor, known to immunologists as IgG, constitutes ~75% of all antibodies found in normal human serum. Healthy B cells have no problem converting IgM to IgG through a process called “class switching,” in which protein tools snip out IgM-specific regions in a DNA strand and then paste in analogous IgG DNA sequences. That maneuver creates recombined genes that express IgG antibodies, which unlike IgM, are capable of neutralizing pathogens, helping other cells recognize invaders (including cancer cells), and maintaining an organism’s well-being.
And that is where TET comes in. Rao’s group found that mutant B cells lacking TET2 and TET3 flip this switch only poorly and instead remain stuck making IgM antibodies because they lack the requisite DNA splicing tool. Specifically, mutant cells lacking TET2 and TET3 do not make enough of a protein called AID, which actually executes the IgM-to-IgG splicing trick, simply because TET2 and TET3 were not available to demethylate and hence enhance expression of the AID gene. In mutant cells lacking TET2 and TET3, the AID gene likely remains methylated, inaccessible and silent, allowing IgM antibodies to predominate.
An inability to perform class switching has clinical consequences: patients who inherit mutations in the AID gene suffer an immune deficiency called hyper IgM syndrome, in which their B cells can’t make the normal conversion of IgM to IgG antibodies. Those individuals are more vulnerable to severe infection and to malignancy.
TET genes have not yet been implicated in hyper IgM syndrome. But the revelation that the AID gene is a TET target makes unquestionable immunological sense to Lio in this context. “TET2 is the most frequently mutated gene in blood cancers including diffuse large B cell lymphomas, which suggests that it restrains cancer progression in normal B cells,” he said. “More importantly, the full activity of TET proteins requires vitamin C. Our study may explain how a healthy diet may enhance our immune response.”
Shukla concurred. “TET proteins are often inhibited in cancers, including cancers derived from B cells addressed here,” he said. “An important rationale for this paper was to identify the normal function of TET proteins so that we better understand why cancers develop when TET genes are restrained.”