Scientists in Israel report that breast tumors can increase their growth by recruiting stromal cells originally formed in the bone marrow. The study (“Bone marrow–derived fibroblasts are a functionally distinct stromal cell population in breast cancer”), published in the Journal of Experimental Medicine, shows that the recruitment of bone marrow-derived fibroblasts lowers the odds of surviving breast cancer, but suggests that targeting these cells could be an effective way of treating the disease.
Within solid tumors, cancer cells are surrounded by other cell types that, though not cancerous themselves, boost tumor growth and metastasis. Breast tumors, for example, contain large numbers of fibroblast cells that promote cancer cell proliferation, inflammation, and the formation of new blood vessels to supply the growing tumor with nutrients and oxygen. Many of these cancer-associated fibroblasts are derived from the neighboring breast tissue, but others seem to come from elsewhere in the body.
Neta Erez, Ph.D., senior lecturer, and colleagues at the Sackler School of Medicine, Tel Aviv University, discovered that, in mice with breast cancer, a significant number of cancer-associated fibroblasts are derived from mesenchymal stromal cells (MSCs). The researchers found that breast tumors can recruit MSCs from the bone marrow and cause them to develop into fibroblasts.
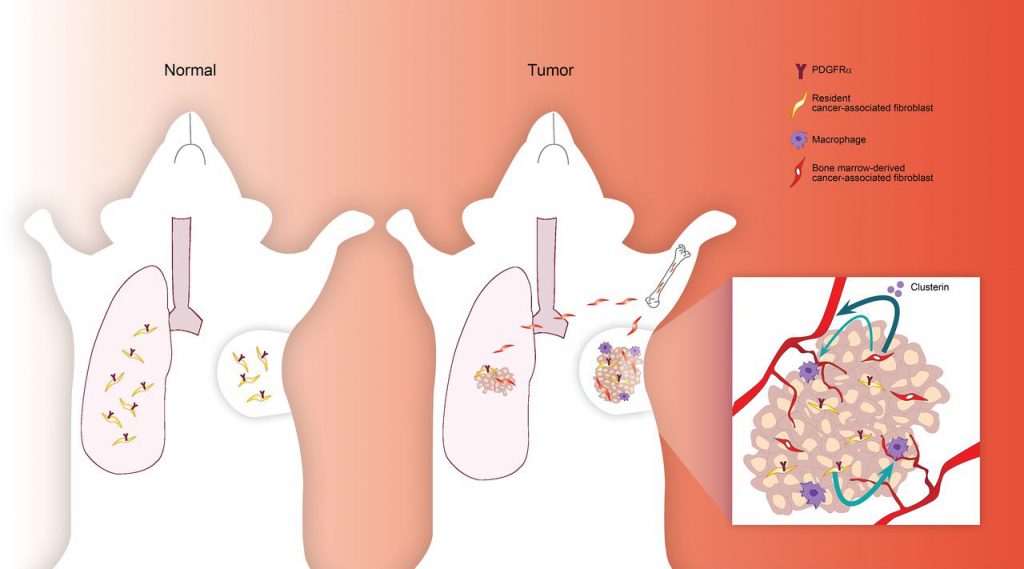
“Cancer-associated fibroblasts (CAFs) are highly prominent in breast tumors, but their functional heterogeneity and origin are still largely unresolved. We report that bone marrow (BM)-derived mesenchymal stromal cells (MSCs) are recruited to primary breast tumors and to lung metastases and differentiate to a distinct subpopulation of CAFs. We show that BM-derived CAFs are functionally important for tumor growth and enhance angiogenesis via up-regulation of clusterin. Using newly generated transgenic mice and adoptive BM transplantations, we demonstrate that BM-derived fibroblasts are a substantial source of CAFs in the tumor microenvironment,” wrote the investigators.
“Unlike resident CAFs, BM-derived CAFs do not express PDGFRα, and their recruitment resulted in a decrease in the percentage of PDGFRα-expressing CAFs. Strikingly, decrease in PDGFRα in breast cancer patients was associated with worse prognosis, suggesting that BM-derived CAFs may have deleterious effects on survival. Therefore, PDGFRα expression distinguishes two functionally unique CAF populations in breast tumors and metastases and may have important implications for patient stratification and precision therapeutics.”
These bone marrow-derived fibroblasts are different from other cancer-associated fibroblasts. They lack, for example, a key cell signaling protein (PDGFRα). But bone marrow-derived fibroblasts are particularly effective at stimulating the formation of new blood vessels because they produce large amounts of clusterin. Tumors containing bone marrow-derived fibroblasts were, therefore, more vascularized and grew faster than tumors that only contained breast-derived fibroblasts.
Dr. Erez and colleagues found that human breast cancers also contain fibroblasts lacking PDGFRα, suggesting that human tumors may also recruit bone marrow-derived cells. Moreover, tumors containing lower levels of PDGFRα tended to be more deadly, suggesting that the recruitment of bone marrow-derived fibroblasts is a crucial step in breast cancer progression.