Post-mitotic neurons in the brain that re-enter the cell cycle quickly succumb to senescence, and this cell cycle re-entry and accumulation of resulting senescent neurons is more common in Alzheimer’s disease (AD) brain than in the normal brain, according to the results of a study headed by Kim Hei-Man Chow, PhD, and colleagues at the Chinese University of Hong Kong. The team applied a bioinformatics approach to analyze single nucleotide RNA sequencing (snRNA-seq) datasets from human AD brain samples. They found that these senescent cells displayed more proinflammatory, metabolically deregulated, and pathology-associated signatures in the AD-affected brains. Through their study the team also found the same general features of cell cycle-re-engaging neurons in a population of dopaminergic neurons in Parkinson’s disease (PD)-Lewy body dementia (LBD), and in a mouse model of brain aging.
The researchers further suggest that the technique they used to make their discoveries will be readily applicable to other research investigating unique populations of cells in the brain. Reporting their results in PLoS Biology “Neuronal cell cycle reentry events in the aging brain are more prevalent in neurodegeneration and lead to cellular senescence,” they noted that the discoveries may one day help scientists identify new biomarkers for diagnosis, or discover therapeutic strategies for relevant disorders. In their published paper the team concluded, “The data that illustrate disease-specific molecular signatures and the new marker genes detected in senescent neurons may also lead to new directions for future diagnosis and development of senotherapeutic strategies.”
Most neurons in the brain are post mitotic, meaning that they have ceased to divide. For many years it had been assumed that this post-mitotic state was permanent. However, recent discoveries have shown that a small proportion of neurons do re-enter the cell cycle. “Increasing evidence indicates that terminally differentiated neurons in the brain may recommit to a cell cycle-like process during neuronal aging and under disease conditions,” the team stated. “While some studies have suggested that the rapid reexpression of cell cycle-related genes in neurons immediately leads to cell death and apoptosis, other reports have indicated that this phenomenon is instead a possible driver of the cellular senescence response.” However, little is known about the fate of these cells. “Because of the rare existence and random localization of these cells in the brain, their molecular profiles and disease-specific heterogeneities remain unclear.”
To address these gaps in knowledge, the authors turned to publicly accessible human brain single-nucleus RNA sequencing (snRNA-seq) datasets. snRNA-seq involves isolating individual single nuclei and sequencing their RNA to provide a snapshot of what the cell was doing at the time of isolation.
The cell cycle proceeds through distinct phases, including growth, DNA synthesis, division-specific growth, and mitosis, and each phase is characterized by a specific set of proteins required to carry it out. This allowed the authors to use the RNA datasets to tell them which phase of the cycle any specific nucleus was in at the time of sequencing.
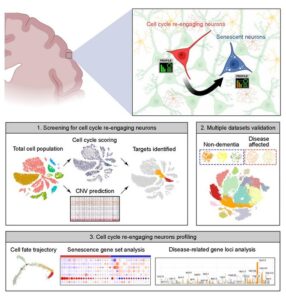
Intriguingly, the authors found that neurons in the brains of Alzheimer’s disease patients reentered the cell cycle at a higher rate than those in the healthy brain, and that those neurons that had re-entered the cell cycle and aged, had increased expression of multiple genes associated with a higher risk of Alzheimer’s disease, including those that contribute directly to production of amyloid, the protein that aggregates in the AD brain. “Quantitatively, the number of cell cycle re-engaging and senescent neurons decreased during the normal brain aging process, but in the context of late-onset Alzheimer’s disease (AD), these cells accumulate instead,” they further noted. “Transcriptomic profiling of these cells suggested that disease-specific differences were predominantly tied to the early stage of the senescence process, revealing that these cells presented more proinflammatory, metabolically deregulated, and pathology-associated signatures in disease-affected brains.”
The team validated its findings in multiple datasets. Similarly, they found that brains from patients with Parkinson’s disease and Lewy body dementia had an increase in the proportion of cell cycle-reentering neurons compared to healthy brains. Tests in a mouse model of brain aging also identified senescent neurons, which exhibited features similar to those identified in the human brain. “These similarities may be useful in explaining how advanced aging may serve as the greatest risk factor for AD and dementia,” the authors suggested.
The neurobiological significance of this heightened cell cycle re-entry for the diseased brain is still unclear, but the analytical approach used by the investigators may offer deeper insights into neuronal subpopulations within the brain, as well as shedding light on disease mechanisms in neurodegenerative diseases. “Taken together, the results of this study illustrated a robust bioinformatics strategy that allows thorough investigation of the molecular profiles of cell cycle re-engaging neurons,” the scientists reported. “The findings here demonstrated that cellular senescence is likely the immediate cell fate of these neurons, which also offered valuable insights into the molecular heterogeneities that could exist among these cells manifested in healthy and disease-affected brain microenvironments.”
The authors acknowledged that further study in human samples will be needed. Chow said, “While experimental validations of these findings in relevant human samples will be conducted in the future, the applicability of this analytical approach in different diseases and cross-species settings offers new opportunities and insights to supplement mainstay histological-based approaches in studying the roles of these cells in brain aging and disease pathogenesis.”
The authors added, “This bioinformatics analytical pipeline demonstrated will offer the field a new tool to unbiasedly dissect cell cycle re-engaging and senescent neurons, and to dissect their heterogeneities in healthy versus disease-affected brains.”