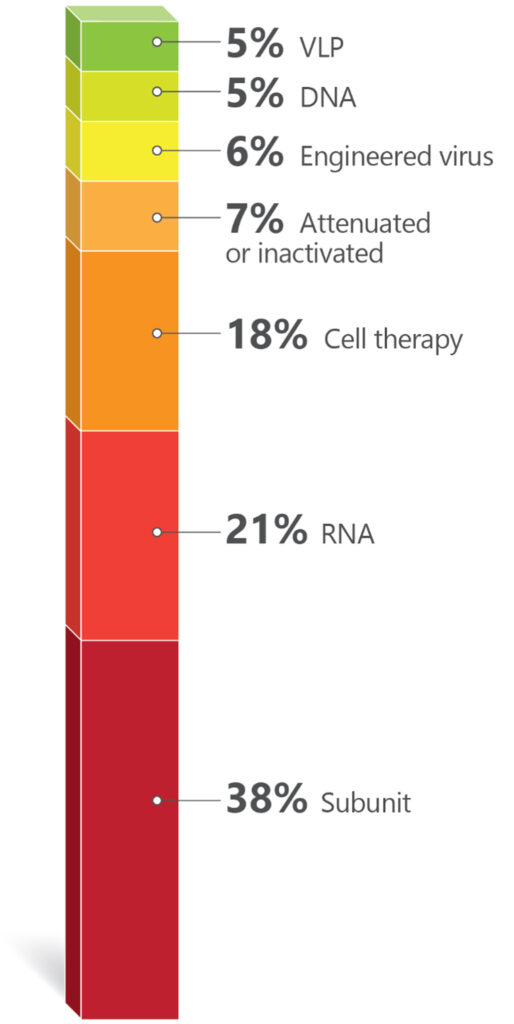
RNA-based immunizing agents have enriched the repertoire of technologies used for vaccine development. An analysis of the active clinical trials in early Phase I and Phase I during the year of 2023 illustrates this point. A total of 161 active vaccine trials were registered at clinicaltrials.gov. Information from these trials was used to plot (Chart 1) and (Chart 2) SARS-CoV-2/COVID-19 trials were excluded to give more evidence to other disease targets.

The commercial success of Shingrix®, the GSK vaccine against Herpes zoster, has led to five companies in China to follow suit with their own products. Moderna is also pursuing the shingles market with an mRNA-based candidate, while Immorna (China) is testing a self-replicating RNA vaccine. Another competitive market is vaccines for respiratory tract infections. RSV is the leading causative agent for lower track respiratory diseases, causing 3.4 million hospitalizations and 950,00–150,000 deaths every year.
Until recently, no vaccines against the virus were commercially available, but two were approved by the FDA in 2023. Arexvy® from GSK, was approved for older adults, and Abrysvo® from Pfizer, for adults and pregnant women (to protect infants from birth to six months). Four other companies are developing vaccines against RSV.
Blue Lake Biotechnology (U.S.) and Codagenix (U.S.) are testing pediatric vaccines. The former is working on an intranasal recombinant parainfluenza virus type 5 for infants and children, and the latter, also developing an intranasal pediatric vaccine, but based on a live-attenuated engineered virus. Sanofi has two trials, one for infants (intranasal) based on live attenuated virus and one for adults (intramuscular) against both RSV and hMPV (human metapneumovirus).
Targeting both these viruses in one jab is also in Moderna’s and AstraZeneca’s pipeline. While Moderna is developing an mRNA-based formulation for infants, AstraZeneca, through the acquisition of Icosavax, has a VLP (Virus-Like Particle) platform technology with a lead program targeting both viruses[1].
Decades of difficulties in the development of vaccines against HIV resulted in many companies dropping their research programs targeting the virus. Of the eight ongoing trials, seven are sponsored by research institutions and only one is sponsored by a company, Vir Biotechnology.
Second largest target
The second largest target in number of trials is cancer (56 trials). The vast majority involve the development of immunotherapies and a recurrent approach are therapeutic vaccines using dendritic cells loaded with personalized neoantigens. Ten out of the 56 cancer trials are based on RNA technologies. Nature’s publication from June 2023[2], showing promising results of a personalized neoantigen mRNA immunotherapy for pancreatic cancer, indicated the feasibility of this complex approach.
The number of companies as sponsors of early-stage trials for vaccines for cancer is smaller than that for viral infections (many cancer trials are sponsored by research/academic institutions). Nevertheless, a good proportion of the company-sponsored trials are for cancers associated with viruses, such as is the case for the HPV vaccines. The vast majority of cervical cancers is associated with the presence of the HPV virus and immunization programs almost eliminated cervical cancer in women born in England since 1995[3].
It is reasonable to hope that the vaccines being developed for EBV (Epstein–Barr virus) or CMV (Cytomegalovirus) associated cancers (hematological malignancies and glioblastomas, respectively) can be successful as well. Immunomic Therapeutics has a DNA formulation based on its UNITE® proprietary technology that explores the CMV-GBM association.
The twelve trials in the protozoa group (Chart 1) are all against just one protozoan, Plasmodium, the causative agent of malaria. Six are sponsored by the University of Oxford. R21, the vaccine developed by Oxford University and the Serum Institute of India, is based on a recombinant protein. It is the second vaccine recommended by the WHO for malaria, a fusion recombinant product which is part circumsporozoite protein of Plasmodium falciparum and part Hepatitis B surface protein antigen.
Recombinant construct
Expressed in yeast, this recombinant construct is more immunogenic and cheaper to manufacture than Mosquirix® (the first malaria vaccine recommended by WHO, developed by GSK)[4]. It is worthy to note that all formulations in University of Oxford’s trials employ the Matrix-MTM adjuvant, which was developed by Novavax and is made with compounds from the bark of the Chilean tree Quillaja saponaria[5].
Twenty trials are registered against bacterial infections: tetanus, diphtheria, Shigellosis, pneumococcal, streptococcal, and meningococcal infections, tuberculosis, and Lyme Disease are the targets. Old unmet medical needs, such as Lyme disease and tuberculosis, are being addressed (also) by mRNA technologies. The Austrian company Eveliqure has a new approach for attenuating genetically engineered bacteria and is testing its oral ShigETEC against Shigellosis. In Australia, GPN Vaccines innovated on inactivation of whole organisms and is using its technology against pneumococcal infections.
Two trials are against Alzheimer disease, both sponsored by research institutions. One is a DNA based vaccine that aims to elicit antibodies against amyloid-β.The other, interestingly, is testing if the BCG vaccine (Bacillus Calmette–Guérin) has an effect on reducing biomarkers for the disease. BCG vaccines are based on attenuated forms of the bacterium Mycobacterium bovis.
The only trial against a fungal infection targets yeast. The goal is to generate protection for women who experience recurrent vulvovaginal candidiasis, and the trial is sponsored by the Swiss company LimmaTech Biologics, in collaboration with GSK. There are no available vaccines against fungal infections, which are responsible for an annual global death rate of approximately 1.5 million[6].
More input needed from South America
Interesting points could be drawn from the analysis of the ongoing early Phase I and Phase I clinical trials on vaccines. There were more European biotechnology companies (11) (Austria, Denmark, France, Germany, Switzerland, Sweden) plus one in the U.K., than there were American companies (10).
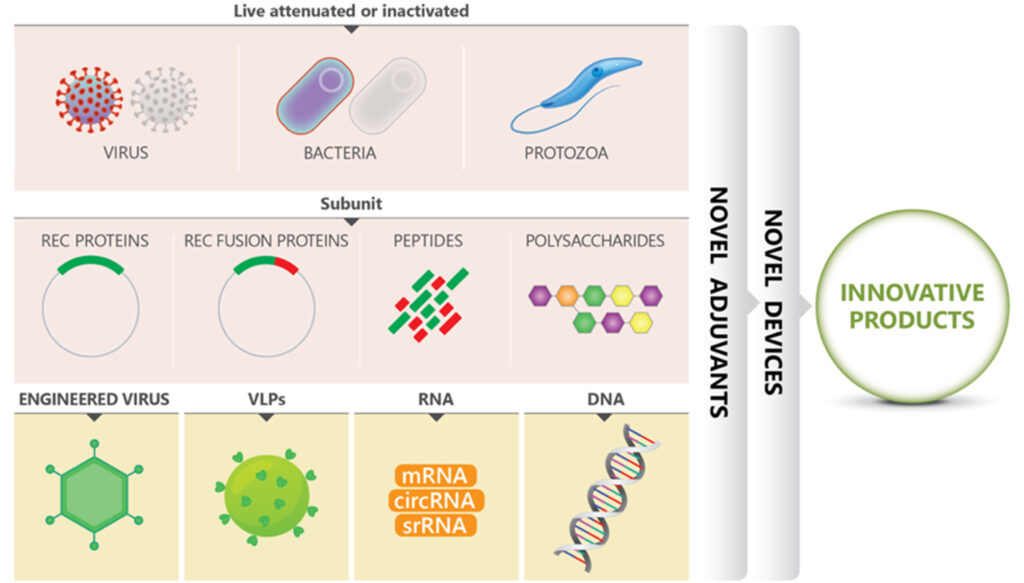
By adding the Australian and Asian companies in early stages clinical trials, there were 30 biotechs dedicated to the development of interesting immunizing agents. By mix and matching old approaches (inactivated or attenuated) with more modern ones (recombinant proteins, fusion constructs, VLPs, RNA, DNA), using systems biology, genetic engineering, AI), innovation in vaccines is flourishing. See Figure 1.
Brazil has an admirable vaccination program and internationally recognized governmental vaccine factories (Butantan and Biomanguinhos), but a poor track record on innovation in this area[8]. It is senseless to invest so much in the training of excellent immunologists, vaccinologists, and infectious disease experts and not translate this into novel products. A lot more can be done.
If it depends on the initiative, CTVacinas (soon to be CN Vaccines, CN for National Center), coordinated by the researcher Ricardo Gazzinelli, DSc, DVM, Oswaldo Cruz Foundation and Universidade Federal de Minas Gerais (UFMG), it will be done.
For those who know Gazzinelli, it is easy to bet that the enterprise under his leadership, which includes the collaboration of several other researchers from UFMG, will thrive. When it comes to partnerships, CTVacinas is open and agnostic; what matters is that meaningful projects move forward, and partners can come from private, public, international or national institutions. An important part of their mission is to be an institute good at translating academic research into novel immunizing formulations. The aim is to generate more businesses, products, and startups.
CTVacinas has a history: part of its revenues come from royalties from a vaccine for dogs against visceral leishmaniasis, developed by Gazzinelli and his business partner, Ana Paula Fernandes.

Helton Santiago, MD, PhD, a professor at UFMG who works with Gazzinelli, is responsible for SpiN-Tec’s clinical program, among others. He is an enthusiastic member of the CTVacinas vaccine team and is optimistic about the future of Brazil in the vaccine field. According to Santiago, the COVID-19 pandemic stimulated the national players to move at a faster pace toward innovation.
If some needed a pandemic to shake them out of a sluggish mode, others have started out going as fast as possible. It is the case, for example, of Luana Raposo, Bruna Porchia, and Mariana Diniz, founders of ImunoTera, a spinoff from the University of São Paulo (Figure 3). With proprietary technology developed during their PhDs, they have succeeded in securing funds for their company’s first steps.

Denise Golgher, PhD ([email protected]) is a life sciences consultant based in São Paulo, Brazil. Rob Rodrigues, LLM, Stanford Law School, is a partner at Licks Attorneys. Many thanks to our interviewees, Luana Raposo from Imunotera, Walderez Dutra from UFMG, Ricardo Gazzinelli and Helton Santiago from CT vacinas, Eduardo Levi from DASA, Jorge Kalil from USP and Constantino Lopez from Instituto Mexicano del Seguro Social.
References:
[1] www.astrazeneca.com/media-centre/press-releases/2023/astrazeneca-to-acquire-icosavax-including-potential-first-in-class-rsv-and-hmpv-combination-vaccine-with-positive-phase-ii-data.html
[2] Rojas LA et al. Nature. 2023; 618:144.
[3] www.thelancet.com/journals/lancet/article/PIIS0140-6736(21)02178-4/fulltext
[4] News in brief. Nature Biotechnology. 2023; 41:1491
[5] www.novavax.com/science-technology/matrix-m-adjuvant-technology
[6] Inácio MM et al. Journal of Fungi. 2023; 9:633.
[7] IQVIA. Race for Immunity: Exploring the Evolving Landscape of the Vaccines Market.2023.
[8] Homma A et al. Vacinas e vacinação no Brasil: horizontes para os próximos 20 anos. Edições Livres. 2020.


