Antibodies perform like guided missiles, inspiring therapeutic developers to produce their own antibody-based weapons systems. Whereas natural antibodies have structures that are determined by the adaptive immune system, designed antibodies have structures that reflect human ingenuity. Ultimately, both natural and designed antibodies do the same thing: recognize pathogenic targets and help bring about their destruction.
Designed antibodies, however, have an advantage. Whereas natural antibodies are typically monospecific, designed antibodies may be multispecific; that is, they may be bi-, tri-, or even tetraspecific. In the target-rich environments presented by disease processes, multispecific antibodies may prove to be effective weapons systems, perhaps even more effective than current antibody-based therapeutics.
More than 100 antibody-based therapeutics have been approved for the treatment of diseases ranging from cancer to neurodegeneration. To build on this success, drug developers are advancing the development of multispecific antibodies. (Most of these new antibodies are bispecific, which means they are able to bind to two different targets at the same time.) Indeed, more than 100 bispecific antibody products are in clinical trials.
Several of the latest bispecific/multispecific antibody technologies are described in this article, which GEN prepared after speaking with several experts in the field of antibody design. For example, there are technologies for developing T-cell bispecific antibodies, which are novel cancer treatment candidates that can crosslink tumor cells and T cells. There are technologies to enhance immunoglobulin (IgG) hexamerization, the formation of six-antibody complexes that can be induced by cell surface antigens. There are technologies to concentrate radioactive therapy by using bispecific antibodies that simultaneously engage a tumor and a small radioactive peptide. Finally, there are technologies to move beyond bispecific antibodies and create multispecific antibodies that can better address the multifactorial traits of diseases.
Neoantigens as beacons
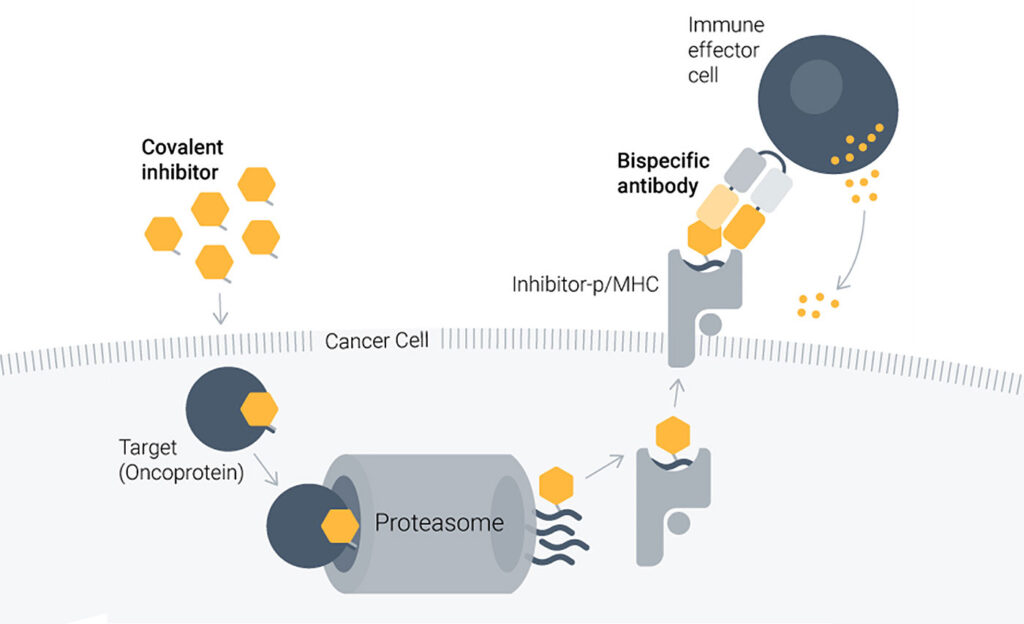
Despite the transforming impact of covalent small-molecule inhibitors, cancer drug resistance remains a daunting challenge. To take on this challenge, Aethon Therapeutics develops antibodies that can enhance the effects of small-molecule inhibitors, or targeted covalent inhibitors (TCIs), by alerting the immune system to TCI-generated neoantigens.
“Aethon unites cancer-specific covalent inhibitors with immunotherapy based on T-cell-engaging bispecific antibodies to make responses to targeted cancer therapies more effective, with a potential for cures,” says Raj Chopra, FRCP, FRCPath, FRSB, PhD, the company’s CEO. (He is also head of oncology and a venture partner at Apple Tree Partners, which has Aethon in its portfolio.)

CEO, Aethon Therapeutics
Aethon’s proprietary HapImmune platform was developed by its scientific co-founders at New York University (Hattori et al. Cancer Discov. 20239; 13: 132–145). “They found that covalent inhibition of target proteins caused by genetic mutations in oncogenes such as KRAS results in the cell surface presentation of a cancer-specific neoantigen drug-peptide on MHC molecules,” Chopra relates. “This MHC presentation of the drug-peptide conjugate is a beacon, if you will, that appears on the tumor cell but not on healthy cells. This beacon exists even when the cell is resistant to the original covalent inhibitor drug.”
Aethon creates specifically engineered bispecific antibodies targeting this drug-peptide MHC complex with high precision. “The beacon presented by the MHC helps the immune system detect and eradicate the persister and resistant cancer cells,” explains Christoph Rader, PhD, the company’s CTO. “It’s a one-two punch in which the first blow is targeted therapy and the second blow by the immune attack elicited by the Aethon drug.”

CTO, Aethon Therapeutics
The company’s proof-of-concept preclinical studies have focused on an oncogenic KRAS mutation frequently found in non-small cell lung cancer (NSCLC) and other cancers treated with the approved covalent inhibitors sotorasib and adagrasib. “To further enhance the covalent inhibitor’s activity, Aethon has developed companion T-cell-engaging bispecific antibodies targeting the drug-peptide MHC complex created by sotorasib and adagrasib,” Rader notes. “[The company] has confirmed their efficacy and selectivity in both in vitro and in vivo models of NSCLC.”
Chopra and Rader say because of its versatility, the platform will be applied to virtually any covalent drug that selectively modifies a cancer mutation and becomes targetable as an MHC-restricted synthetic neoantigen on the cancer surface.
Hexamer technology
Utilizing multiple antibody design approaches can expand therapeutic applications. “We have developed several technology platforms—including the DuoBody, HexaBody, DuoHexabody, and HexElect platforms—to generate novel antibody-based therapeutics that are designed to harness the power of human antibodies,” says Esther Breij, PhD, vice president, global head of translational research, Genmab. “[Our goal is to transform] how cancer and other serious diseases are treated.”

VP, Global Head of Translational Research, Genmab
The DuoBody platform generates bispecific antibodies suitable not only for discovery but also for commercial manufacturing scale using a sequential process. The process involves initial production of two IgG1 antibody molecules of the desired specificity that contain matched mutations in the constant domain (Fc domain). Following separate expression and purification, the two antibodies are recombined under a process called controlled Fab-arm exchange.
The HexaBody platform modifies monoclonal antibody to enhance target-mediated IgG hexamerization on the plasma membrane. “Combining these technologies can allow for the creation of even more potent therapeutics,” Breij suggests. “We are basically combining dual targeting with enhanced potency to increase antibody effector functions.”
The utility of the technology has been demonstrated in recent preclinical studies. For example, Genmab researchers and their colleagues used it to develop DuoHexaBody-CD37, a humanized bispecific IgG1 antibody that shows promise against B-cell malignancies (Oostindie et al. Blood Cancer J. 2020; 10: 30). This antibody binds two non-overlapping epitopes on CD37 with an engineered hexamerization-promoting mutation in the Fc region.

CD37 is a tetraspanin selectively expressed on B cells. Most monoclonal antibodies specific for CD37 are poor inducers of tumor killing by complement-dependent cytotoxicity. However, DuoHexaBody-CD37 showed efficient cytotoxicity-mediated tumor cell killing in primary tumor cells from patients with B-cell malignancies, and in leukemic cell lines and patient-derived xenograft models representing different B-cell malignancies.
Genmab has used the DuoBody platform as the basis of four approved bispecific antibody medicines. The company has about 20 products in clinical development that incorporate hexamer technology.
Safer radioimmunotherapy
Treating cancer with radioimmunotherapy involves the use of tumor-targeting antibodies that are labeled with radioisotopes. “However, the exposure of healthy tissue to radiation has been a major challenge,” advises Randolf Kerschbaumer, PhD, the CEO of OncoOne. “We have engineered our proprietary technology to address the challenges of antibodies directly linked to a cytotoxic payload, especially cytotoxic radionuclides, thereby minimizing the risk of healthy tissue exposure.”

CEO, OncoOne
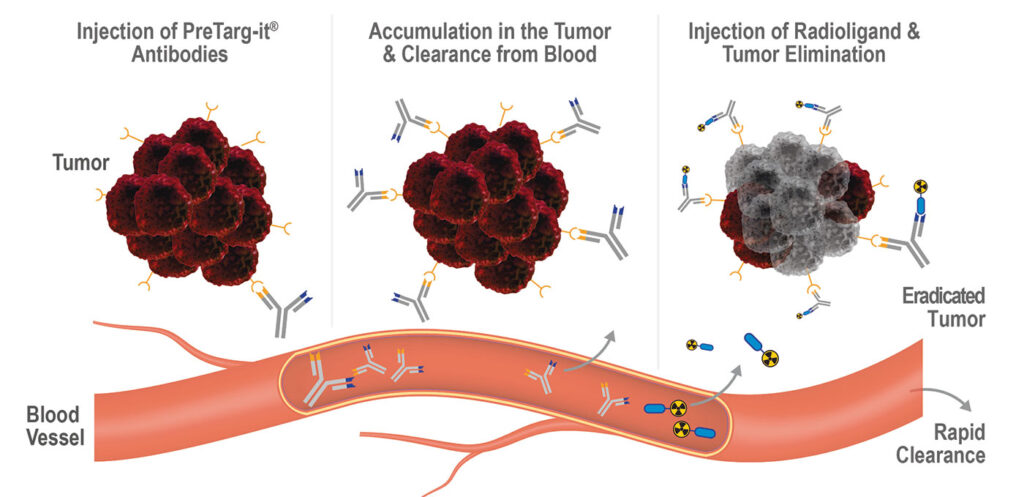
“Our PreTarg-it platform is a two-component system for the pretargeted delivery of radionuclides to the tumor,” he continues. “The first component is a tailored bispecific antibody, in which one binding arm has a high specificity for a tumor target. The bispecific, nonradioactive antibody is applied first and accumulates at the tumor site expressing the target while unbound antibodies are cleared from the circulation.
“Once the bispecific antibody is cleared from circulation, a small peptide that is conjugated to a chelator loaded with a radioactive nuclide is applied. The peptide is then trapped in the tumor via the antibody’s second specificity, while the excess of unbound radioactive peptide is excreted within hours through the kidney and bladder. Thus, the cytotoxic activity is almost exclusively concentrated in the tumor.”
According to Kerschbaumer, the PreTarg-it platform has demonstrated its versatility and modularity by helping OncoOne generate four therapeutic candidates. “The bispecific antibody consists of modules that can be exchanged, thereby allowing us to generate bispecific antibodies capable of binding to various tumor targets,” he explains. “We can also conjugate peptides to different cytotoxic agents, like radionuclides, immunomodulators, and (encapsulated) toxins. Therefore, our platform enables us to target different tumor antigens with payloads of different substance classes.”
Currently, the PreTarg-it platform is being investigated in hard-to-treat cancers such as pancreatic cancer. “These diseases usually have a very poor prognosis and are often too far advanced when diagnosed to respond to conventional approaches such as immunotherapy,” Kerschbaumer observes. “We are advancing the platform currently through preclinical evaluation, where we have already demonstrated its ability to deliver strong efficacy within one treatment cycle.”
Multispecifics to enhance potency
“Even though patients have a single disease, it may display multifactorial traits,” says Mark Chiu, PhD, president and CEO, Tavotek Biotherapeutics. “Thus, we engineer our molecules to have multiple mechanisms of action.” Tavotek accomplishes this task by using its multispecific antibody platform.
“While bispecific antibodies have two recognition arms, multispecific antibodies have more than two engaging domains,” Chiu points out. “The purpose of both antibody types is to accomplish multiple functions as mediated by interactions with disease targets.”
Chiu adds that a trispecific antibody may have two arms that bind cancer targets and a third arm with a unique function such as immune cell engagement to then kill the tumor cells. “Tavotek utilizes a repertoire of modules—including antibody-based binding arms, cytokines, and enzymes—to engineer multispecific antibodies with avidity that exploit dominant traits of multifactorial diseases to enhance therapeutic drug efficacy.”
According to Chiu, the design of bi-, tri-, tetra-, or multispecific molecules has the following steps: First, define a therapeutic mechanism of action. Second, generate the building blocks. (For this step, Tavotek has the TavoSelect platform.) Third, assemble the building blocks into a differentiated molecule with unique functions to generate the building blocks. (For this step, Tavotek has the TavoPrecise platform).
Tavotek is developing TAVO412, a multispecific antibody that is in a Phase I study for the treatment of advanced or metastatic solid tumors. TAVO412 targets mesenchymal-epithelial transition factor (cMET), epidermal growth factor receptor (EGFR), and vascular endothelial growth factor (VEGF).
Cancer often progresses because of the development of mutant forms of EGFR and enhanced crosstalk with cMET, remarks Maria MacWilliams, PhD, vice president of molecular biology at Tavotek. “TAVO412 pairs anti-EGFR and anti-cMET with anti-VEGF binding arms to enhance tumor resistance mitigation and shut down angiogenesis,” she adds. “This ‘trifecta’ approach extends TAVO412’s use to patients suffering from triple-negative breast cancer or from liver, gastric, or pancreatic cancer.”
Tavotek has oncology drug candidates in addition to TAVO412 in its pipeline. Also, the company is developing immunology drug candidates. Finally, as Chiu notes, the company has plans to develop multispecific antibodies that enable specific delivery of siRNA to diseased tissue. Possibilities include the control of master regulator gene expression, targeted engagement to activate or modulate immune cells, and intracellular targeting agents that can cross tissue barriers to facilitate various kinds of delivery (such as oral, pulmonary, and cerebral delivery).