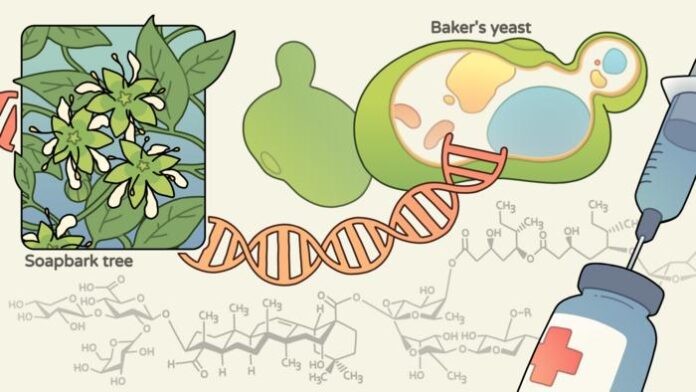
Vaccine adjuvants are molecules or compounds that prime the immune system to mount a more robust defense against infection. While they are added to vaccines—such as the Novavax COVID-19 vaccine—in small quantities, adjuvants can have a big protective effect, particularly in infants with immature immune systems, or in older people with declining immune responses.
One of the strongest adjuvants, an extract of the Chilean soap bark tree (Quillaja saponaria), is so difficult to produce that it costs several hundred million dollars per kilogram. Scientists at the University of California (UC), Berkeley, and Lawrence Berkeley National Laboratory (Berkeley Lab), working with researchers at the John Innes Center in the U.K., now report that they have harnessed synthetic biology to produce the active ingredient of soap bark, a molecule called QS-21, in yeast.
Production of QS-21 in this way involved the insertion of 38 different genes from six organisms into yeast—building one of the longest biosynthetic pathways ever transplanted into any organism, said Jay Keasling, PhD, UC Berkeley professor of chemical and biomolecular engineering and senior faculty scientist at Berkeley Lab.
While yields from the yeast-based process are still small—a few hundred dollars’ worth from a liter of broth—the achievement promises to make one of the most effective adjuvants available more broadly and to lower the cost of vaccines in general. Producing these types of compounds in yeast is not only cheaper, but more environmentally friendly, avoiding many of the caustic and toxic chemicals needed to extract the compound from plants. The yeast-based process could also be harnessed to help researchers develop improved vaccine adjuvants.
“During the pandemic, public health officers were really worried about QS-21 adjuvant availability because that only comes from one tree,” noted research lead Keasling. “From a world health perspective, there’s a lot of need for an alternative source of this adjuvant.” Former UC Berkeley postdoctoral fellow Yuzhong Liu, PhD, now an assistant professor at Scripps Research in La Jolla, CA, added, “The production of the potent vaccine adjuvant QS-21 in yeast highlights the power of synthetic biology to address both major environmental, as well as human health challenges.”
Liu is first author of the team’s published paper in Nature, titled “Complete biosynthesis of QS-21 in engineered yeast,” in which the scientists concluded, “… the yeast biosynthetic platform provides vast opportunities to produce structural variants of QS-21 by expressing alternative pathway enzymes or by making fragments of QS-21, exploiting the promiscuity of the enzyme in the pursuit of new leads for vaccine adjuvants.”
Adjuvants increase the efficacy of vaccines by stimulating or augmenting the human immune response to pathogens or disease-specific antigens, the authors explained. Because adjuvants make vaccines more effective, they also allow doctors to use smaller doses of antigen. The benefit of adding an adjuvant to a vaccine was first noted in the 1920s, when it was discovered that alum—the aluminium salt aluminium hydroxide—could boost the effectiveness of a diphtheria vaccine. Alum has since been added to many vaccines that use a portion of a pathogen to induce immunity and is, the team noted, the most widely used, clinically approved vaccine adjuvant.
Not long after alum was discovered to boost the effectiveness of vaccines, a group of soap-like molecules was found to do the same. By the 1960s, researchers had focused on an extract of the Chilean soapbark tree that strongly activates different components of the immune system to amplify the effect of giving a vaccine antigen alone. For the last 25 years, one component of that extract—QS-21—has been one of the main non-aluminum adjuvants in vaccines, having been tested in more than 120 clinical trials. It is found in the shingles vaccine (Shingrix) given to older adults, a malaria vaccine (Mosquirix) currently used in children to protect against the parasite Plasmodium falciparum, and the Novavax COVID-19 vaccine.
However, the authors noted, “Despite major commercial interest, the availability of QS-21 remains limited, owing mainly to its structural complexity.” QS-21 is produced by stripping bark from the soapbark tree and chemically extracting and separating its many compounds, some of which are toxic. “Traditionally, QS-21 is extracted from the tree bark of the soapbark tree Quillaja saponaria, which is native to Chile,” the authors explained. “Isolation is complicated because the plant extract contains a multitude of different structurally related Quillaja saponins, rendering the purification process highly laborious and low yielding.” This traditional method of extraction and purification also destroys the tree bark, prompting increased governmental regulations around its deforestation.
In fact, the complex QS-21 molecule contains a terpene core and eight sugar molecules, and while it has been synthesized in the laboratory, this synthesis takes 79 separate steps, starting from an intermediate chemical that itself has to be synthesized. “… the overall yield is negligible,” the authors noted. “Thus, developing alternative production processes that are more sustainable and scalable would help to meet the ever-increasing demand for potent vaccine adjuvants, and to address existing or emerging medical needs.”
Keasling, who is CEO of the U.S. Department of Energy-funded Joint BioEnergy Institute (JBEI), was asked to try to recreate the synthesis process in yeast because he has worked for years adding genes to yeast to get them to make terpene compounds, among them the antimalarial drug artemisinin, but also scents and flavorings. Terpene compounds, like those responsible for the scent of pine trees, are often fragrant.
“This work builds on our malaria work,” Keasling explained. “We worked on the malaria therapy. Now, this could be an adjuvant for the malaria vaccines in the future.”
The team said that adding the eight sugars proved challenging, as did balancing unsuspected interactions among enzymes in yeast. All this had to be accomplished without throwing off critical metabolic pathways that are needed for yeast growth. “It has eight sugars and a terpenoid in the middle. I mean, it makes the artemisinin biosynthetic pathway look like nothing,” Keasling pointed out.
Keasling and lab colleagues, led by Liu, worked closely with plant researcher Anne Osbourn, PhD, at the John Innes Center. Osbourn had earlier teased out the many enzymatic steps involved in the soapbark tree’s production of natural QS-21. Over the past five years, as Osbourn discovered new steps in the process and tested them in tobacco plants, Keasling’s lab gradually added these new genes to yeast to replicate the synthetic steps.
“It was a great collaboration, because as soon as she’d get a new gene in the pathway, they’d send it our way, and we’d put it into yeast,” Keasling said. “It was also good for her, because she got a test of whether her tobacco assay was telling her the right thing.”
Earlier this year, Osbourn and Keasling published the complete 20-step process by which the soapbark tree makes QS-21, reconstituted in tobacco. Unfortunately, tobacco is a test bed for plant chemistry, but not a scalable way to produce a chemical compound.
The newly reported achievement switches that process to yeast, with additional steps because yeast do not contain some enzymes that naturally exist in plants. Currently, a liter of the fermenting bioengineered yeast can produce about 100 micrograms of QS-21 in three days, with a market value of about $200. “Even at the levels we’re producing it, it’s cheaper than producing it from the plant,” Keasling said. “I am gratified that synthetic biology has come so far that we can now build a pathway to produce a molecule like QS-21. It’s a testament to how far the field has progressed in the last two decades.”
The authors added, “Our demonstration of the total biosynthesis of QS-21 in an engineered yeast strain highlights the possibility of replacing the plantation-based supply of saponins with industrial fermentation at scale, which could markedly increase the availability of QS-21 to meet the rising demand for potent vaccine adjuvants.”
At present, the yeast strain used produces less QS-21 than the w/w yield from the tree, the team noted, but it does so over a period of days. “As a result, the production of QS-21 in yeast is still considerably faster (by approximately 1,000 times) than it is in the native Q. saponaria, which produces QS-21 in trees only once they reach an age of 30–50 years.”
Yeast biosynthesis is also scalable, the researchers pointed out, and the engineered yeast subsists only on sugar, which is an added advantage, Keasling noted. “My whole thing is, I want to make everything from a single sugar. I just want to feed yeast glucose, because eventually we want this process to be scaled. And if you feed them a bunch of fancy intermediates, then it’s going to result in a process that is not scalable,” Keasling said. “In the end, I’d like to start with glucose, so when the production is performed in large tanks, they’re able to produce QS-21 as easily and inexpensively as possible.”
The authors acknowledged that further key developments in strain engineering, production, and fermentation schemes, as well as in the downstream extraction and purification processes, will be needed to produce yeast-derived QS-21 at scale. Nevertheless, they stated, “… landmark successes in this arena, such as the industrial-scale production of the anti-malarial precursor artemisinic acid, have paved the way for new opportunities in microbial biomanufacturing.”
While Keasling plans to leave optimization of the yeast-based production process for large-scale production of QS-21 to others, he does hope to tweak the enzymatic steps he has introduced into yeast to produce variants of QS-21 that could potentially be more effective than QS-21. Yeast biosynthesis allows him to experiment with pruning the QS-21 molecule to see which portions can be eliminated without altering the molecule’s effectiveness. As the team concluded in their paper, “Owing to the promiscuity of several enzymes, structural analogues of QS-21 were produced using the biosynthetic platform described here; this will enable a structure–bioactivity relationship to be established in the future, and will allow the rational design of potent vaccine adjuvants.”

