Sponsored content brought to you by
DNA plays an important role in cell and gene therapies (CGTs), diagnostics, agriculture, and the development of novel technology. To date, CGT research and clinical applications have typically used viral vectors as delivery agents. While viral vectors, especially AAV, have shown their value, immune issues and payload limitations exist. Plus vector manufacturing is costly and complex, slowing development and impacting the cost of therapies. Is nonviral delivery an alternative route?
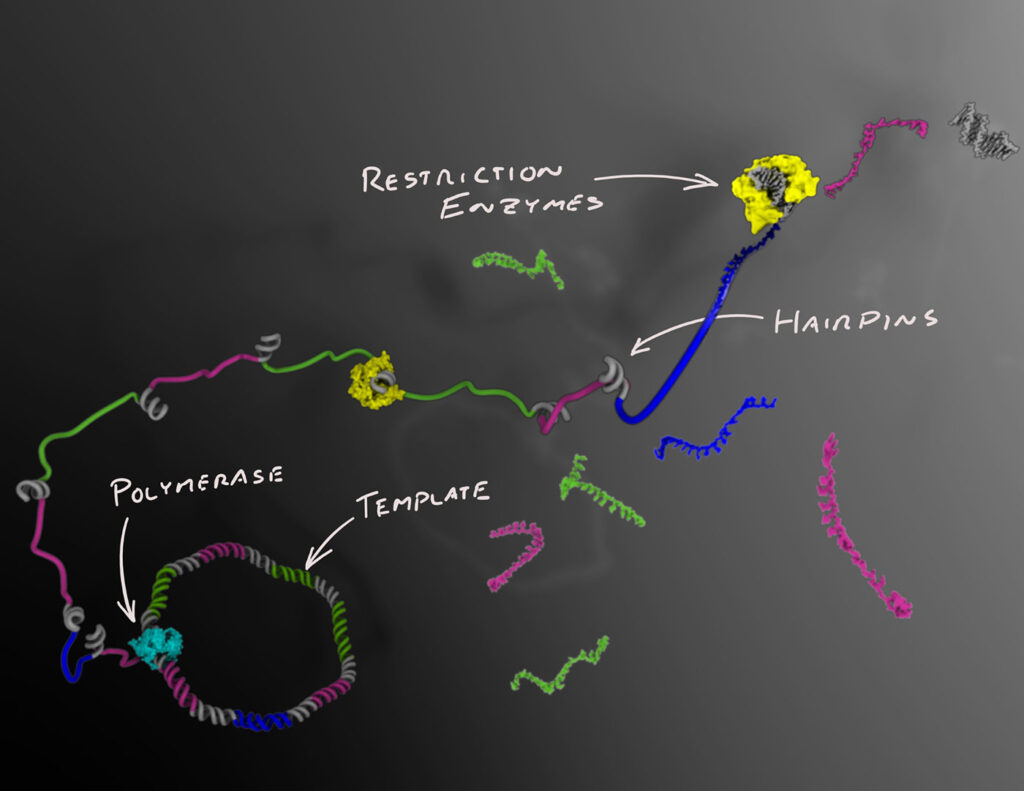
An Alternative Route
Moligo Technologies is collaborating with Nationwide Children’s Hospital to provide long, single-stranded DNA (ssDNA) for its research into gene therapies for cystic fibrosis and other airway diseases. Using ssDNA to insert large genes, such as CFTR (cystic fibrosis transmembrane conductance regulator), opens up new opportunities for CGT.
“If we can successfully use ssDNA to insert large genes, such as CFTR, into airways, the possibilities are endless. There are few options outside of AAV right now in the genome-editing field,” said Sriram Vaidyanthathan, PhD, a principal investigator at Nationwide Children’s Hospital Center for Gene Therapy at the Abigail Wexner Research Institute. “Although the efficiency of editing and producing ssDNA capable of carrying long genes has been a challenge, Moligo’s ssDNA has several characteristics that make it a possible option—including its length, ability to be produced at scale, and the fact that it can be chemically modified to potentially improve the stability of the gene.”
The research team has tested the ssDNA in vitro in cultured airway stem cells. The ssDNA templates enable gene insertion. Efficiency is now being optimized.
ssDNA versus dsDNA
Non-viral genome targeting has many potential advantages but some approaches, like dsDNA, have challenges. The toxicity of naked dsDNA limits clinical applications with large non-viral DNA templates. For example, if stem cells are modified using dsDNA, they will launch an innate immune response and often not survive the editing process well.
This toxicity has facilitated a movement towards the use of ssDNA, which is significantly less toxic. As early as 2018, a process for therapeutic gene editing in human T cells was published in Nature that demonstrated that after ssDNA was integrated into primary human T cells the cells remained viable, expandable, and functional.1
Advances continue. A recent 2023 Nature Biotechnology article showed that ssDNA homology-directed repair (HDR) templates incorporating Cas9 target sequences (CTSs) with reduced toxicity boosted knock-in efficiency and yield by an average of around two- to threefold relative to dsDNA CTSs. By enhancing HDR, the collaborative team could further increase knock-in efficiencies. The method worked across a variety of target loci, knock-in constructs, and primary human cell types, reaching HDR efficiencies of greater than 80–90%.2
A Stem Cell Example
“Gene correction is particularly important in the field of exvivo gene therapy and requires using an engineered nuclease and a DNA repair template to edit the genome of long-term hematopoietic stem cells (LT-HSCs),” said Julien Valton, PhD, Vice President of Gene Therapy, Cellectis. However, this cellular subset is highly sensitive to the presence of foreign dsDNA and is thus incompatible with conventional gene correction approaches.
To overcome such sensitivity and enable efficient gene correction in LT-HSCs, Cellectis successfully substituted dsDNA with ssDNA repair templates. According to Valton, their highly encouraging results demonstrate that incorporating ssDNA repair templates in nuclease-mediated gene correction or gene insertion processes holds great promises for the field of exvivo gene therapies.
ssDNA Advancements

Founder and CEO, Moligo
Moligo utilizes a patented, PCR-free “injection molding,” enzymatic technology to produce ultrapure DNA strands over 10,000 bases long, 100% sequence-verified, and at scale. The process also enables any sequence to be synthesized, overcoming complexity-related barriers.3
“To use DNA at large scale you need a lot of high-quality material,” said Cosimo Ducani, PhD, CEO & Founder, Moligo. “We can produce, with high fidelity, molecules which include sequence complexity such as repeats, high GC content and secondary structure. ” We can also synthesize them highly modified, fine-tuning their properties based on the downstream applications.”
References
1. Roth, TL, Puig-Saus, C, Yu, R et al. Reprogramming human T cell function and specificity with non-viral genome targeting. Nature 559, 405–409 (2018). doi: 10.1038/s41586-018-0326-5
2. Shy BR, Vykunta VS, Ha A et al. A. High-yield genome engineering in primary cells using a hybrid ssDNA repair template and small-molecule cocktails. Nat Biotechnol. 2023 Apr;41(4):521-531. doi: 10.1038/s41587-022-01418-8
3. Ducani C, Kaul C, Moche M, Shih WM, Högberg B. Enzymatic production of ‘monoclonal stoichiometric’ single-stranded DNA oligonucleotides. Nat Methods. 2013 Jul;10(7):647-52. doi: 10.1038/nmeth.2503

For more information about Moligo Technologies, please visit www.moligotechnologies.com.



