Drastic differences in the ways cells behave and respond to drugs are observed when cells are cultured using 3D instead of 2D approaches. These differences are driving the adoption of more advanced cell culture models. With the added benefits of 3D cell culture systems come additional challenges in terms of optimization, handling, and assaying. Here, we discuss a number of these obstacles and recommend how they may be addressed.
Spheroid formation
Optimizing the spheroid seeding density, media formulation, or culture period, or adding an overlay of Corning Matrigel® matrix, can lead to tighter spheroid formation.
Spheroid size
Spheroid size is determined by the cell type, seeding density, and culture time. As the spheroid becomes larger, it becomes more difficult for nutrients and oxygen to reach the center of the spheroid, which can lead to a hypoxic core. Depending on the assay, this may or may not be desirable. Optimizing the seeding density will play a very large role in the ability of the spheroids to initially form, as well as determine how long the spheroids can be kept in culture.
Media formulation
Media formulation may affect spheroid formation, and options should be tested with each cell type. Supplements, such as methylcellulose, can increase the viscosity of the medium and have been shown to aid in spheroid formation.
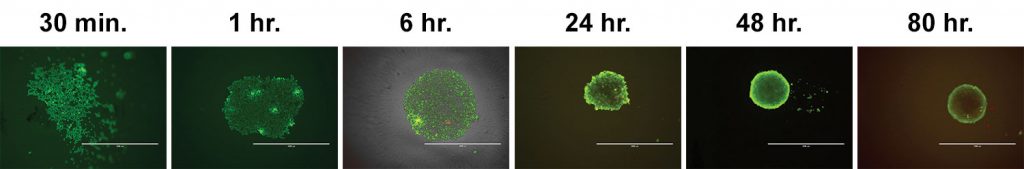
Time to form spheroid
Some cells will readily form spheroids within hours, while other cells will require several days. Monitoring spheroid formation over time will help in determining the ideal culture period for each application (Figure 1).
Spheroid handling
Media and buffer exchanges
Media and buffer exchanges can be challenging when working with spheroids. The process can be time consuming and pose a risk of losing or disturbing spheroids.
Corning spheroid microplates are automation friendly and make media and buffer exchanges easier to accomplish without the risk of disturbing the spheroid. For manual exchanges, we recommend careful pipetting to remove most of the medium in the well, ensuring pipette tips do not scratch the bottoms or sides of the wells. At least 10- to 20-µL residual volume should be left behind to avoid disrupting the spheroids. It is also possible to do half changes of medium more frequently instead of full exchanges.
The BioTek MultiFlo™ FX is an automated multimode reagent dispenser for 6- to 1536-well microplates. When equipped with BioTek’s AMX™ automated media exchange module, the dispenser can provide gentle aspiration and dispensing of media for 3D cell culture. Figure 2 compares spheroid loss from manual versus automated media exchanges. A significant number of spheroids were lost during manual exchanges, as denoted by the columns outlined in red; none were lost using the automated and highly efficient MultiFlo FX system.

Spheroid transfer
Spheroids can be removed from the spheroid microplates using Corning’s Axygen® wide-bore (1–200 µL) tips (Corning Cat. No. TF-205-WB-R-S) or a Corning 5-mL Stripette™. This can be helpful for spheroid-embedding protocols, neurogenesis assays, or any other application in which the spheroids need to be removed from the spheroid microplate.
Centrifugation
Cells seeded in the spheroid microplate can be centrifuged briefly at 300 × g to aid in spheroid formation or centering spheroids when an extracellular matrix is required.
Removal of spheroids from matrix
For applications that require the spheroid to be embedded in Corning Matrigel® matrix, there are several options to recover the spheroid from the matrix. One option is to reduce the temperature of the microplate to liquefy the matrix so that the spheroid can be removed. Another option, depending on the concentration of the matrix, is to add a cold buffer or medium to the well to dilute/liquefy the Matrigel matrix. This may need to be repeated to free the spheroid. Additionally, Corning Cell Recovery Solution (Corning Cat. No. 354253) may be used. It depolymerizes the Matrigel matrix and can be used to recover the spheroids.
Single-cell recovery
For assays requiring single-cell suspensions, spheroids can be dissociated by incubating with reagents such as Corning Accutase®; 5 mM EDTA; 1× trypsin/EDTA; or 1×, 5×, or 10× TrypLE™.
Spheroid assays
The black sidewalls and clear bottom of the spheroid microplate make it an ideal option for numerous fluorescent, luminescent, and colorimetric assays that can be conducted directly in the spheroid microplate. Depending on the size of the spheroid, it may become difficult for some assay reagents to penetrate fully. Therefore, optimization of each assay is recommended using appropriate positive and negative controls.
Homogenous assays
There are several commercially available 3D-specific reagents that have been optimized for use with spheroids. We recommend CellTiter-Glo® 3D cell viability assay (Promega Cat. No. G9683) for enumerating total ATP content of spheroids.
Imaging 3D cultures
Because the spheroid is significantly smaller than the microwell in which it resides, it is critical to understand where the signal is coming from in the well—whether it is a concentrated area or multiple areas within the well. A plate reader is designed to capture as much light from each well as possible, and with spheroids, a pinpoint of light will get lost in the background. Fortunately, with microscopy and image analysis, the field of view can be limited to mostly the spheroid and avoid background coming from the well.
Staining a 3D structure may require protocol optimization compared to the 2D equivalent. In general, the larger and tighter the spheroid, the longer and more complex it will be for complete staining to occur. If cell permeabilization is required, reagent choice and length of incubation time may need to be considered. We have had success using a variety of stains, including primary and secondary conjugated antibodies, with cells cultured in the spheroid microplate. You can also prelabel cells prior to seeding in the spheroid microplate or use fluorescent-protein-expressing cells to ensure that all cells are labeled as needed for the application.
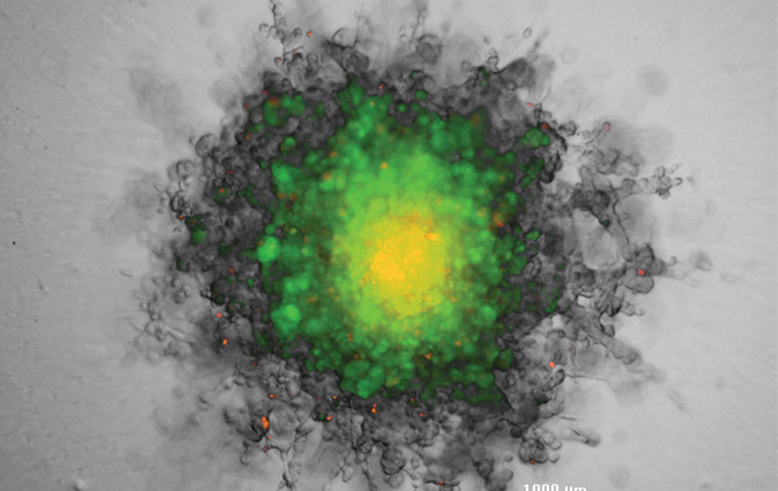
The thickness of 3D cultures also necessitates the ability to image through the structure. The imaging system must be capable of z-stacking—in which the focal height can be changed and a series of images can be taken through the spheroid. A series of z-stacks through the biology can then be assembled into a composite using software algorithms to keep the in-focus portions of the image and reject the out-of-focus portions (Figure 3).
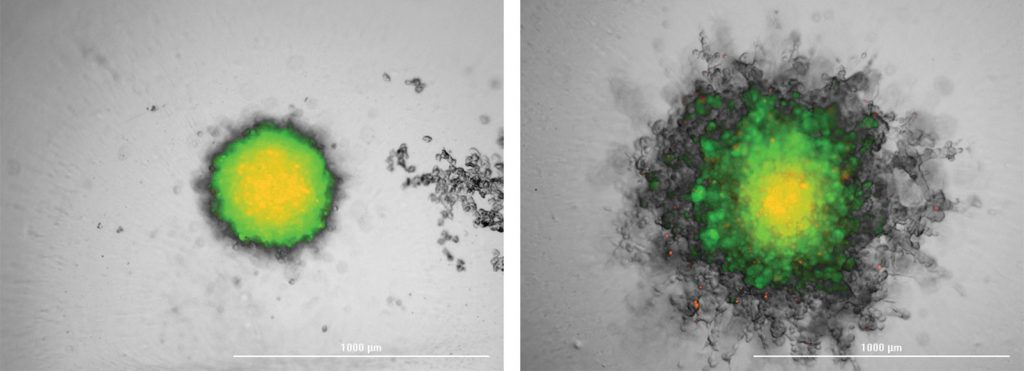
RFP-Fibroblast aggregated into a spheroid, then encased in Matrigel.
Overlaid brightfield and fluorescent images derived from a projection of a 20-slice z-stack, before (left image) and after (right image) a five-day incubation period (BioTek Cytation™ 5).
There is growing interest in adopting 3D culture approaches in drug discovery. Advanced technologies and guidelines for developing and culturing 3D models are increasingly available, making this approach more feasible, efficient, and automated.