September 1, 2007 (Vol. 27, No. 15)
Patricia F. Fitzpatrick Dimond Ph.D. Technical Editor of Clinical OMICs President of BioInsight Communications
Optimizing Transient Systems to Save Time and Money
Researchers and companies are devising promising approaches to circumvent limitations of existing transfection technologies. Ongoing challenges, particularly with regard to high-quality recombinant human protein production, include cell-type specific requirements for most methods, length of time and cost of creating stable transfectants, and the need for serum-free culture conditions.
New approaches in development include optimization of transient systems to reduce time and costs associated with stable transfectant development. Gene transfection constructs containing human genetic elements that can insulate transfected genes of interest from repressive effects related to insertion-site loci are also becoming available. At the “Protein Expression Europe” meeting to be held later this month in Prague, several presentations will cover these developments.
Michael Dyson, Ph.D., head of the protein expression subgroup of the Atlas of Protein Expression Project at the Wellcome Trust Sanger Institute, will discuss the development of a small-scale transient transfection expression screen of suspension mammalian cells to rapidly identify transfectants and perform optimizations. By coupling small-scale multiplate culture and fluorescent dot blot analysis of transfection supernatants, Dr. Dyson and his team were able to rapidly analyze protein expression on a small scale, obtaining results that correlated well with larger-scale protein expression and purification.
Rapid Transfectant Screening
According to Dr. Dyson, dot blot analysis of transfection supernatants yielded similar results as Western blot analysis and provided good correlation between the results of the plate-based small-scale transient transfection results and proteins levels expressed at the 50-mL scale.
This is the first published report of a 24-well block prescreen validated for secreted proteins using the cost effective polyethylenimine (PEI) as a transfection reagent, says Dr. Dyson. “The relatively inexpensive PEI transfection reagent we describe allows a greater number of constructs and/or expression conditions to be examined in parallel. Our group at Sanger Institute has recently performed 384 transfections in a single day. We believe it is now feasible to rapidly screen multiple variations in expression conditions, and this could be very useful for secreted antibody expression optimization.”
Dr. Dyson describes another potential application in the generation of receptor protein microarrays for use in functional ligand screening and antibody specificity assays. The method uses GATEWAY-adapted expression vectors and a HEK293E suspension cell line developed by Yves Durocher of the animal cell technology group of the Biotechnology Research Institute in Montreal. At the Prague meeting, Dr. Dyson will describe transient infection of HEK-293E cells grown in suspension culture with a nonredundant set of 526 mammalian cell-surface receptor domains using GATEWAY expression vectors.
The aim of the Atlas of Protein Expression Project at the Sanger Institute, headed by John McCafferty, co-inventor of antibody phage display and a founder of Cambridge Antibody Technology, was the high-throughput generation of antibodies to mammalian cell surface receptors for expression mapping using immunohistochemistry. However, following a change in strategic focus at the Sanger Institute to concentrate on human and experimental genetics and pathogen biology, the Atlas Project is now in the process of being wound down and relocated to the department of biochemistry, University of Cambridge, U.K., to apply its technology to the area of cell-cell communication.
Selexis’ (www.selexis.com) goal has been to rapidly identify highly efficient stable transfected producing cells through introducing, along with the transgene and promoter, proprietary genetic elements says Igor Fisch, Ph.D., president and CEO.
High-producing Clones
The company’s DNA Elements are human genetic elements that effectively insulate transfected genes from local negative influences of chromatin that may repress the expression of the transgene. “This approach,” says Dr. Fisch, “has allowed us to get a much higher proportion of cells that have incorporated the transgene and to rapidly identify high-producer stable transfectant clones without prolonged amplification procedures. Selexis Genetic Elements work by increasing copy number-dependent, position-independent gene expression.”
Since transgene expression varies greatly among independent transformants, identification of high-producer clones requires lengthy selection, screening, and subsequent amplification procedures. Variability in expression usually results from the number of transgenes incorporated and the site of chromosomal integration.
For example, gene expression may be silenced if integration close to a repressed chromatin segment occurs. One way in which Genetic Elements are thought to increase the number of high-producing transfectant cells is by organizing chromosomal domains into structures permissive or non-permissive for gene expression. A transgene flanked by such elements may remain protected from, for example, the repressive effects of histone acetylation.
“Our Genetic Elements, once integrated, reorganize the DNA structure around the locus of transgene integration. So, even if the transgene is integrated into a transcriptionally inactive site, the elements remodel the DNA to make it transcriptionally active,” says Dr. Fisch.
He says that the Genetic Elements expression technology can increase recombinant protein expression in mammalian cells in suspension and in serum-free more than 20-fold and within about five weeks of transfection, high-producer clones were identified after screening no more than 200 clones. The Selexis system allowed the integration of 50–100 transgene copies into a single chromosomal locus, preferentially near cellular telomeres resulting in extremely stable cell lines.
“One of our recent clients was able to rapidly create a new, CHO-based stably transfected cell line producing high levels of IgG4 where they were not previously able to do so,” according to Dr. Fisch.
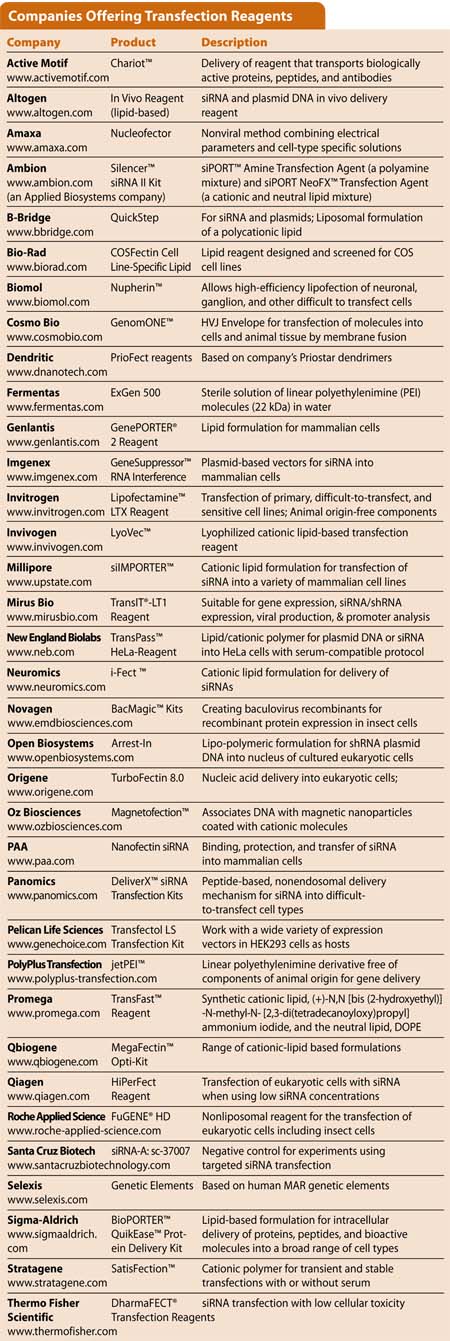
Companies Offering Transfection Reagents
Reagent Toolbox
Scott Pattison, Ph.D., director of molecular biology at Kemp Biotechnologies (www.kempbiotech.com), says that the need to develop novel reagents and processes for their contract research service projects has led to the ongoing development of a complete toolbox of reagents, which are used for production of recombinant proteins in mammalian cells.
Acquired by Pelican Life Sciences in 2006, Kemp Biotechnologies is a contract cell culture, molecular biology, protein expression, and purification services provider. Many of the tools that Kemp Biotechnologies uses for transient mammalian cell protein production are being converted into products to be sold by Gene Choice (www.genechoice.com), another Pelican Life Sciences company.
“We had spent a great deal of time finding ways to use transient instead of stable transfection to produce recombinant proteins and thought that others could benefit from our efforts,” Dr. Pattison says. When protein production clients came to us requiring protein expression in mammalian cells we were at one time confined to using stable cell lines. These lines generally take at least four months to produce and characterize. Three years ago we asked ourselves—how viable would a transient expression platform be for these clients? By successfully developing a reliable transient process, we could realize significant time and cost savings in delivering a protein.”
Kemp scientists came up with a lipid-free reagent for transfection and developed a HEK293-based cell line that could grow well in serum-free suspension culture. “We have repeatedly delivered in excess of a gram of purified protein to our clients in eight weeks or less (from microgram quantities of plasmid to purified protein), using our transient expression process, compared to the four or five months generally required for stable cell line development,” he says.
Gene Choice now markets Transfectol™-LS Transfection Kits, which the company says work with a wide variety of expression vectors in HEK293 cells as hosts. Proteins produced using this transient system have included secreted proteins such as Fc fusions, intracellular proteins including kinases, and cell surface proteins such as ion-channel proteins and GPI-anchored proteins. The company is working on adapting CHO and NSO cell lines as acceptable hosts for large-scale transient production.
Dr. Pattison describes a protein production challenge illustrating the time and cost benefits of Kemp’s transient mammalian expression process. “A client came to us requesting that we produce one gram of recombinant immunoglobulin. The original process that they had developed at their facility used adherent serum-containing cultures of a HEK293-derived cell line. Their procedure involved transfecting multiple batches of 50 T180 flasks of these cells, utilizing a lipid-based transfection reagent.
“While reasonable product yields were achieved, the process was an extremely labor-intensive and costly approach. We were able to apply our bioreactor-scale transfection process in which we could transfect the same number of cells (~2e9 cells) in a single 20-L serum-free culture, with our Transfectol-LS transfection reagent. The final mean volumetric yield for the project was 15 mg of purified product per liter of cell culture. It took us six weeks to deliver 1.1 grams of their purified IgG, and the product met the client’s endotoxin and bioactivity specs. Implementing our bioreactor-scale process cut the number of hands-on research scientist hours needed to complete the project at least in half, compared to the original protocol.
“Utilizing a serum-free process minimizes concerns over adventitious agents, is a major cost-saver, and makes life much more pleasant for downstream protein recovery and purification.”
Dendritic Nanotechnologies (www.dnanotech.com), a subsidiary of Starpharma Holdings, entered a license and supply agreement with EMD Biosciences in February for its PrioFect™ Transfection reagents based on the company’s Priostar dendrimers. Dendritic Nanotechnologies says that its dendrimers are well-defined, highly branched synthetic macromolecules synthesized by surrounding a core particle with layered branching elements produced through a proprietary synthetic process.
According to Lori Reyna, Ph.D., senior scientist and manager of life sciences, “What distinguishes our dendrimers is that we have unique control over the size of the nanodendrimers we produce.”
Citing issues including the lack of ability of existing reagents to infect a variety of cell types, poor targeting, and toxicity, the company says its dendrimer production technology affords unique size control in nanometer increments, thereby allowing optimization of siRNA delivery into individual cell lines. “While we don’t at the moment clearly understand why we are able to transfect a variety of cell lines using our dendrimers between two and tennanometers, depending on the cell type, we do know we can greatly increase transfection efficiencies through our ability to control dendrimer size.”
Dendrimers are synthesized as a single molecular entity with high structural and chemical homogeneity and offer a precisely controlled macromolecular surface, according to Dr. Reyna. The company says that such dendrimers can be used in an “uncapped” state or functionalized by conjugation of active groups to its surface for use as potential pharmaceuticals as well as targeted nucleic acid transfection reagents.
“A lot of the development work we have done is for commercialization for siRNA and DNA transfection platforms. But because of the dendrimer structure, we can add properties such as nuclease protection, a very important characteristic. We can also target the dendrimers by adding small molecules or antibodies,” explains Dr. Reyna. The size of the particles, she says, also allows passive targeting, for example, in in vivo nucleic acid delivery applications, because nanodendrimer biodistribution varies based on size.
In a pharmaceutical application, Starpharma’s VivaGel, a vaginal microbicide, is based on the antiviral properties of a lysine-based dendrimer with naphthalene disulphonic acid surface groups and has been successfully tested in Phase I clinical trials for the prevention of HSV-2 infection. The product is currently being tested in an expanded Phase II trial in Kenya, Puerto Rico, and San Franciso.
Off-target Effects
According to Mike Leous, marketing manager at Roche Applied Science (www.roche-applied-science.com), Roche hopes to make researchers using transfection techniques aware that all transfection reagents induce some off-target effects (significant up or down regulation of expression levels of genes not targeted by the transfected sequence) even in the absence of a vector insert. “Sometimes these effects kill the cell; other times they can have a significant impact on the experiment and overall research project,” Leous says. “We believe that researchers should consider these off-target effects and we fear that many scientists are unaware of them.”
As a case in point, Leous cites a study by Roche in which scientists evaluated expression profiles of two human cell lines transfected with two different plasmids using Roche FuGENE® HD Transfection Reagent or another reagent from a different supplier.
In one experiment in which MCF7 cells were transfected with control plasmid, FuGENE HD Transfection Reagent led to a total of 197 genes up or down regulated, while the comparator reagent affected 1,405 genes.
In both MCF7 cells and HeLa cells, FuGENE HD Transfection Reagent altered expression of a relatively small number of genes involved in biological process, such as DNA-dependent transcription, compared to the other reagents, which altered at least twice as many genes affecting important biological processes. “Our focus in improving transfection processes is developing innovative transfection reagents that minimize off-target effects,” Leous adds.
Patricia F. Dimond, Ph.D., is a life science
consultant. E-mail: [email protected].