March 1, 2008 (Vol. 28, No. 5)
New Methodology Takes the Development of Soluble Receptor:Fc Fusion Proteins Forward
Expression of cytokines or growth factors and their subsequent binding to cell surface receptors in healthy individuals is a tightly regulated and coordinated process. The inappropriate expression of these circulating proteins can contribute to the onset or progression of a number of acute and chronic diseases.
One method for treating diseases that result from overexpressed cytokines or growth factors is to block their ability to bind to their cognate cell surface receptor. A common strategy to achieve this is to use mAbs that specifically target these proteins. An alternative strategy is to use a soluble version of the cognate receptor itself to bind to and block the action of the cytokine.
Soluble receptors are often expressed in mammalian cells as a hybrid or chimeric molecule consisting of the extracellular domain of the cell-surface receptor fused to the Fc portion of a human immunoglobulin (Ig). Such fusion proteins, when expressed, are designed to dimerize, in a manner analogous to the dimerization of the Ig heavy chain, to create a bivalent, soluble receptor capable of blocking the action of cytokines and growth factors. This approach is exemplified by the anti-inflammatory drug Enbrel®, which is a fusion of the extracellular domain of the TNF-a receptor with the Fc portion of human IgG1.
Low productivity levels and/or poor product quality beset the expression of some soluble receptor-fusion proteins when expressed under similar conditions as mAbs. Low expression levels will necessitate either increased manufacturing capacity or increased facilities time to deliver sufficient amounts of material to satisfy clinical or commercial demands. High levels of aggregate may necessitate the need to develop additional chromatography steps, which can greatly reduce the overall yield and drive up the overall process time and facilities costs.
Despite these problems, soluble receptors may remain an attractive alternative to mAbs. Cell surface receptors have evolved over time to bind with high specificity to their cognate cytokine or growth factor and are therefore already optimized for use as a blocking reagent.
Identifying and developing a therapeutically useful mAb is often a long and costly process (with additional time and costs if humanization of the mAb or other alterations are required). If the sequence information has been determined for the receptor, it may be relatively straightforward to design a soluble form of the receptor and have confidence that it will retain the desired binding characteristics. The speed and relative ease of designing soluble receptors enables more rapid, in vitro, proof-of-concept studies to be completed and can potentially accelerate the timeline for introducing the molecule into the clinic.
There may also be additional business drivers for choosing to produce a soluble receptor over a blocking mAb. Clearly, strategies for improving the expression and quality of these soluble receptor-fusion proteins may contribute to the success of bringing these molecules forward into the clinic.
A Problem with Misfolding
The low productivity of cell lines expressing soluble receptor-fusion proteins as well as the poor product quality of these proteins may be due to poor protein folding. While consideration may be given to the design of the soluble receptor-fusion protein at the primary amino acid level, secondary or higher-order structures may not be optimal for facilitating the proper folding or structural stability of the soluble receptor-fusion protein, with consequences to both expression and product quality.
The folding stability of proteins can be reflected in their thermal-melting profile as determined by differential-scanning calorimetry (DSC). A protein that exhibits its first thermal transition at temperatures that are well above typical cell culture temperatures (i.e., >37ºC) is likely to adopt a stable folding conformation and will be well expressed.
Most mAbs, for example, display their first thermal transition at 60ºC or greater. In contrast, proteins whose structures display a thermal transition at temperatures at or near the cell-culture temperature are less likely to adopt a stable folding conformation and as a result, are often poorly expressed and are prone to aggregation. Lowering the cell-culture temperature may promote more stable protein-folding conformations and should result in more efficient transit through the secretory pathway.
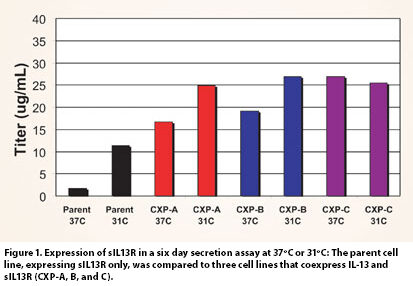
This phenomenon was observed during the development of a soluble IL-13 receptor: Fc fusion protein (sIL13R) at Wyeth BioPharma. The DSC profile of sIL13R indicated a thermal transition at approximately 40ºC. When cultured at 37ºC, the expression levels of sIL13R were low, but the expression was greatly enhanced by reducing the cell culture temperature (Figure 1, Parent).
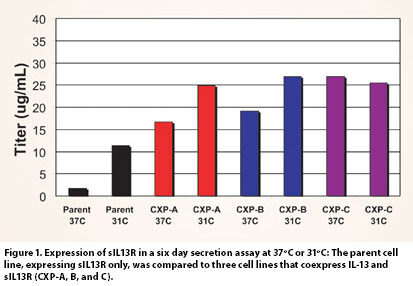
Figure 1
The increased production of sIL13R at lower temperatures occurs downstream of transcription and translation, as Northern blot analysis and pulse/chase experiments indicated that there was no change in the levels of sIL13R specific transcript or primary translation product as a result of temperature shift. Subcellular fractionation experiments confirmed that at the lower culture temperature, sIL13R moved more rapidly through the secretory pathway. This data together suggests that the lower culture temperatures promote or stabilize a more favorable folding conformation for sIL13R, which allows it to be secreted efficiently from cells after translation.
This data also predicts that the product quality of the secreted protein should be improved at lower temperatures, as properly folded proteins are often less prone to aggregation. Indeed, the levels of sIL13R high molecular weight (HMW) species, or aggregates, are significantly reduced when cells are cultured at lower temperatures (Figure 2, Parent).
What if culturing cells at reduced temperature isn’t enough, or worse yet, doesn’t work at all for a candidate soluble receptor-fusion protein? Indeed, while HMW levels were greatly reduced for sIL13R in the low-temperature process, the overall levels remained >10% and the process still required expensive chromatography steps for HMW removal.
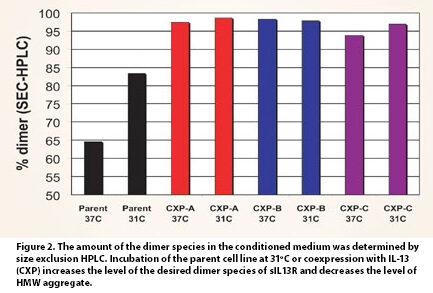
Figure 2
It may be possible to lower the culture temperature further in an attempt to increase the stabilization of protein folding. This, however, would likely also suppress cell growth and limit cell-mass accumulation during production, resulting in low process yields. Clearly, alternative methods for stabilizing protein-folding conformations would be desirable.
The role of ER chaperone proteins in facilitating the folding and secretion of proteins has been extensively studied, and attempts have been made to coexpress these proteins in recombinant cell lines. This approach, however, has not been universally successful and in some cases, has resulted in decreased expression of some recombinant proteins.
Interestingly, when sIL13R is coexpressed with its cognate cytokine, IL-13, a dramatic increase in expression beyond that which is seen for the parent cell line even with a temperature shift is observed (Figure 1). The increased expression of sIL13R was not observed when coexpressed with a different cytokine (IL-6), nor was it observed when IL-13 was added exogenously to the cell culture media.
These observations suggest that when coexpressed with the soluble receptor-fusion protein, the cytokine may act as a scaffold, which guides the fusion protein to adopt a stable folding conformation and holding it there as it transits through the secretory pathway and is secreted into the media.
Not only was the expression of sIL13R greatly enhanced by coexpression with IL-13, but the levels of HMW species were also dramatically reduced, both at 31ºC as well as at 37ºC (Figure 2). Thus, while the low yields of sIL13R in the parent cell line may be sufficient to support the needs of a research assessment, the coexpression of IL-13 with sIL13R has enabled the development of a cell-culture process that is more compatible with a manufacturing program (Figure 3).
For the coexpression strategy to be successful as a means to bring soluble receptors into the clinic, the cytokine must first be removed from the soluble receptor. Because the interaction between receptor and cytokine is typically quite strong, purification of the soluble receptor-fusion protein may be difficult.

Figure 3
To overcome this, it may be possible to coexpress mutant forms of the cytokine that exhibit lower binding affinities for the receptor. Such a strategy was successfully applied to cell lines expressing sIL13R. Mutant forms of IL-13 were identified that preserved the ability to enhance productivity and reduce aggregation of sIL13R when coexpressed but could also be dissociated from the receptor under relatively mild wash conditions during chromatography.
Importantly, the purified sIL13R remained properly folded and exhibited acceptable stability and full biological activity. The final process, both cell culture and purification, is nearly identical to those designed for mAbs and fits well into existing facilities. By coexpressing the soluble receptor with a mutant form of its cognate cytokine, we have created a viable path for moving the soluble-receptor program forward.
Gene W. Lee, Ph.D., is director of cell line development at Percivia. Dr. Lee was formerly employed at Wyeth BioPharma, where the study described in this article was performed.
E-mail: [email protected].