April 1, 2013 (Vol. 33, No. 7)
Online Measurement of DO with Chemical Optical Sensors
In bioprocess development for the production of pharmaceuticals, proteins, or nutritional additives, scale-down models are used to save time and costs when testing for suitable cell lines, media composition, and process conditions.
However, it is often difficult to determine critical parameters for scale-up of processes at small scale. Shake flasks are commonly used in biotech research and production. They represent a low-cost alternative to expensive larger-scale bioreactor systems and are a lot easier to handle.
On the other hand there is no established system for online monitoring and control of important culture parameters at this scale. A reliable method for automated feeding and the elimination of manual sampling is needed to improve bioprocess development at shake-flask scale.
In these experiments, a feedback-controlled system was tested that could be applied to shake-flask cultures. A feed with carbon source was triggered by the DO content in the cultures and measured online with chemical optical sensor technology from PreSens.
The oxygen sensors were integrated at the bottom of glass Erlenmeyer flasks and read out noninvasively through the transparent flask wall. The sensor signal was transferred to the OXY-10 mini, a 10-channel oxygen meter allowing simultaneous online measurements in up to 10 shake flasks. Using peristaltic pumps the glucose solution was added to the cultures automatically on demand.
Tests with pulsed feed on demand in cultures of Saccharomyces cerevisiae were carried out and results compared to values gained at bioreactor scale (20 L). Furthermore, the system was used to test a carbon-limited process and to enhance biomass and product yield, where the goal was to keep DO at about 20%.
Materials & Methods
Experiments were carried out in 500 mL glass shake flasks with 100 mL working volume and at a shaking rate of
The Coaster is an accessory that allows transferring the sensor signal to the oxygen meter. The OXY-10 mini was connected via RS232 to a Lantronix S2E-Converter, which was connected to an AdamBus A/D-Converter, which controlled the peristaltic pumps for feeding.
A PC with Lucullus PIMS was used to gather data from the Lantronix S2E-Converter and monitor the process. Each shake flask was connected to an individual peristaltic pump with silicone or pharmed tubing (12–16 tube diameter) allowing a flow of 0–50 g/h. The tubes were attached to the shake flask lids (Figure 1).

Figure 1. Set-up inside the shaker: Shake flasks placed in clamps on top of the Coaster CFG to read the sensor signal; each flask lid is connected to individual peristaltic pumps via silicone or pharmed tubing for carbon feed.
The actual feed rate was calculated by means of correlating flow rate vs. pump speed. First, experiments with four different strains of S. cerevisiae were carried out in duplicate in eight shake flasks. A glucose solution (500 g/L) was given to the cultures on demand as a carbon source with pulses of 0.5 hours at a feed rate of 20 g/h. Feed rate and pulse time are experience values.
Feed start was triggered when DO/10 min was >10 %. In a second experiment, they attempted to keep S. cerevisiae at static DO in a carbon limited process. 150 g/L glucose solution was used and feed start triggered again when ΔDO/10 min was >10%. A simple two-step controller was used to keep DO at a setpoint of 20% with 0.5% increment.
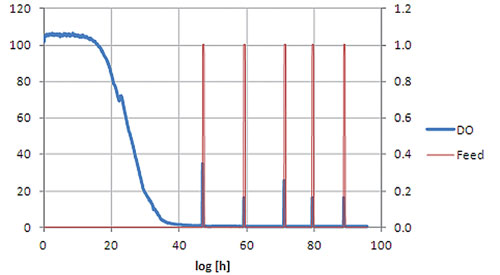
Figure 2. Cultivation of S. cerevisiae in the feed-on-demand shake flask set-up.
Results
The feed-on-demand experiment was run for 96 hours (Figure 2). In the first phase the initial glucose is consumed and due to the diauxic growth of S. cerevisiae partly converted to ethanol. The growth of the cells leads to a decrease in DO level. At around 22h (small peak in DO signal) glucose is depleted and growth continues on ethanol.
Due to the limited oxygen transfer capacity of shake flasks the DO level reaches zero at around 40h. When ethanol is also depleted, no other carbon source is present in the medium, resulting in reduced oxygen uptake by the cells and therefore an increase in DO level. This increase is used as a trigger for glucose addition.
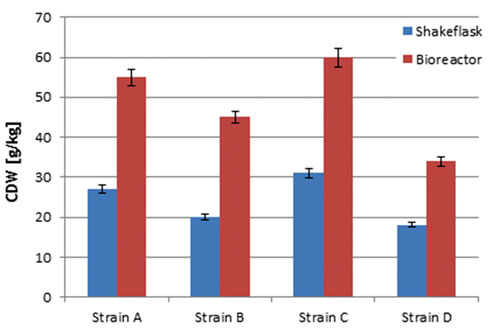
Figure 3A. Comparison of the four S. cerevisiae strains at shake flask scale (blue) and bioreactor scale (red): Cell dry weight.
As soon as the added glucose and the produced ethanol are consumed again, the next addition is started. Comparing dry weight and production yield of the four S. cerevisiae strains in the shake-flask setup with results gained for the same strains at bioreactor scale (20 L), it turned out that the shake-flask cultures reached lower output in cell dry weight and product concentration (Figures 3A–B), which is caused by the higher oxygen transfer capacity of bioreactors.
Nevertheless, all strains showed the same ranking at both cultivation scales, which enables picking strains with special characteristics for later scale-up processes.
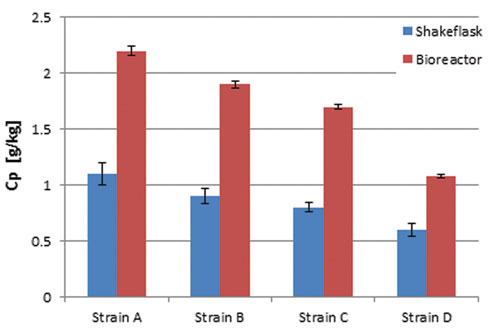
Figure 3B. Product concentration
Comparison of relative production parameters revealed good alignment of the shake-flask culture values with those taken at bioreactor scale (Figure 3C). In a second experiment the automated shake-flask system was tested in carbon-limited cultivation with static DO. Figure 4 shows that the automated feeding system could be used to perform DO-Stat cultivation.
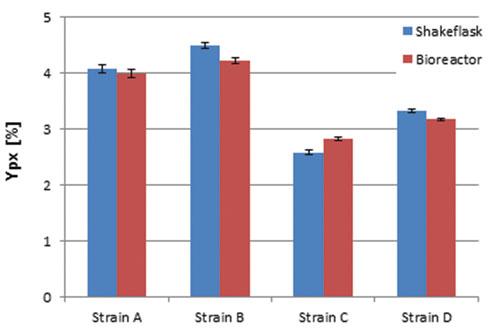
Figure 3C. Relative production parameter Ypx
When DO levels fall below setpoint, feed-rate is decreased and vice versa. The chosen parameters (0.5% feed-rate increment, 1h step time) already resulted in good DO control. Further fine tuning with defined process conditions can lead to even lower oscillation in the DO signal.
The developed feed-on-demand system for shake-flask cultivation showed noteworthy results and, compared with values from bioreactor scale, one can conclude that different strains can be compared and picked at this scale.
Moreover tests in this setup were extremely time saving. Where only two or three experiments can be run at bioreactor scale, this systems allows conducting 8–10 tests a week. Even in a run with a carbon limited process the system performed with unexpectedly good results.
Being able to measure DO online and in several shake flasks simultaneously with the PreSens OXY-10 mini is an important advantage, when conducting experiments in scale-down processes. Additional experiments with the SFR Shake Flask Reader that allows for oxygen and pH measurement in up to nine shake flasks might enhance this automated and partly controllable system at shake-flask scale. Further development of the set-up is already being done.
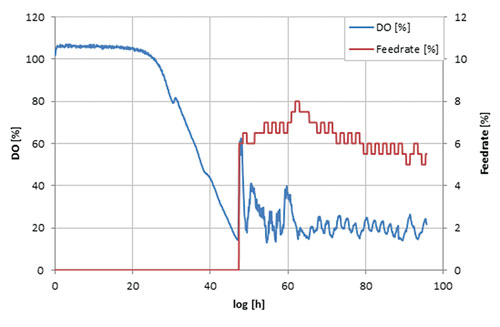
Figure 4. DO-Stat: Carbon-limited process; DO-setpoint 20%.
Manuela Senn and Martin Ischebeck work at DSM Nutritional Products. The OXY-10 mini and SFR Shake Flash Reader are manufactured by PreSens.



