April 15, 2011 (Vol. 31, No. 8)
Ellyn Kerr
Emerging Platforms Afford Timelines More Comparable to Those for Soluble Targets
GPCRs constitute the largest class of known targets in commercial drug discovery today—collectively the targets of at least 30% of currently marketed drugs. Yet, as heptahelical transmembrane proteins involved in divergent and manifold signaling pathways, they can confound conventional drug discovery techniques.
The recent “Congress on G Protein-Coupled Receptors in Drug Discovery” meeting highlighted key approaches from companies pursuing developments in biased agonism, structure elucidation for ligand prediction, allosteric modulation, and advancement of GPCRs from preclinical to clinical studies, particularly with small molecule drug candidates.
Guido Zaman, Ph.D., who heads assay development and pharmacology support for GPCR and enzyme projects at MSD/Merck, presented data on low molecular weight β–arrestin biased ligands of a class B GPCR, the parathyroid hormone receptor PTH-R1.
“Identification of small molecule drugs for GPCRs is not trivial,” Dr. Zaman said, citing a general paucity of structural information on most GPCRs. Further, class B GPCRs, whose native activating ligands are proteins and large peptides, can prove particularly recalcitrant to pharmacological intervention by small molecules.
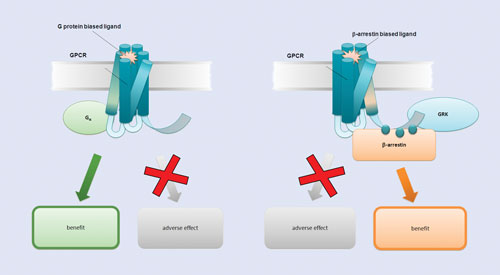
Dr. Zaman’s lab and others are focused on bypassing these challenges by means of biased ligand binding, specifically through manipulation of β-arrestin. Because β-arrestin attenuates G protein coupling to GPCRs either by binding-site occlusion or by promoting cellular internalization of the GPCR, it is a key intervention point in GPCR-related drug discovery.
Binding of either β-arrestin or G protein will be associated with discrete downstream signaling cascades; depending on the particular disease pathway or side-effect profile, either could be desired. As Dr. Zaman noted, in the case of PTH, some models in the literature have shown that an anabolic (tissue-building) response is mediated by β-arrestin, and an osteoclast (bone-degrading) pathway is mediated through G proteins.
Precise and informative assays are thus critical to understanding these complex interactions such that they may be harnessed for effective pharmaceutical development. Dr. Zaman explained that MSD has refined a two-part assay system that confirms biased β-arrestin signaling recruitment: a live-cell, high-content green fluorescent protein imaging assay and a beta-galactosidase enzyme fragment complementation assay. The former permits study of the receptor in nonmodified form; the latter allows cost-effective, high-throughput measurements on standard chemiluminescence readers, he remarked.
MSD has developed an in silico modeling approach that Dr. Zaman said is predictive of small molecule interactions with GPCRs, for in silico selection of compound libraries. Such developments may contribute to a move away from ultrahigh-throughput screening while allowing for increased specificity, always a key concern with any drug candidate but of particular importance with GPCRs given their involvement with numerous signaling pathways.
MSD is using these approaches to develop low molecular weight compounds whose effects mimic those of natural hormones. Its most advanced project in this area, focused on gonadotropins, is currently in the clinic.
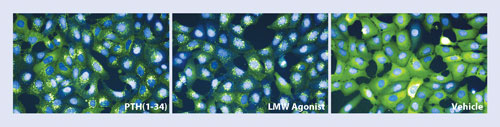
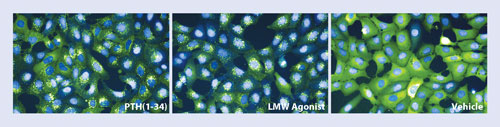
Osteoblasts expressing a fusion protein of green fluorescent protein and ß-arrestin and the PTH receptor were incubated with the natural hormone PTH (left), the low molecular weight agonist (middle), or vehicle control (right). [MSD/Merck]
Trevena is focused on GPCR therapeutics, specifically identification of ligands that bias either β-arrestin or G protein signaling. The company applies its Advanced Biased Ligand Explorer (ABLE™) platform, comprising assay tools and techniques for identifying and pharmacologically characterizing signal-selective ligands.
“Drugs that are marketed today typically turn the receptor on or off, like a light switch,” said Michael Lark, Ph.D., CSO and svp of research. “In actuality, the biology is much more complicated than that. The µ-opioid receptor, for instance, has a side-effect profile associated with β-arrestin that includes respiratory suppression and gastrointestinal side effects; we’re working to identify a ligand that selectively favors the G protein pathway, which induces analgesia without classical opioid side effects.” That project is currently at the lead-optimization stage.
In February, Trevena announced a Phase IIa trial with its lead candidate, TRV120027, a β-arrestin-biased angiotensin II type 1 receptor (AT1R) ligand to treat acute heart failure. Using its platform to characterize the compound, the company noted that the biased ligand favors increased cardiac performance due to its β-arrestin activation, while also lowering blood pressure due to its inhibition of G protein activation. Further, the compound also improves renal perfusion while preserving renal function.
“When we formulate our hypotheses, we’re always thinking about what benefit we can harness by biasing the signaling one way or the other,” Dr. Lark explained.
Once initial hits are identified, assays to clearly functionally characterize their activity at a cellular level are paramount. As Dr. Lark noted, appropriate pharmaceutical activity must obviously be validated in the cell and whole animal: “Does the compound change cellular signaling in the way we predicted? How does it change receptor trafficking and downstream signaling? What biological consequences are associated with this biased ligand?”
All this can provide critical mechanism-of-action information early on in the pipeline efforts. And GPCR drug companies are finding success with approaches based on strong biology and robust data interpretation. Trevena, for instance, was able to screen 11 receptors (from a Ligand Pharmaceuticals five-million-compound library) within an 18-month time frame, and Trevena’s lead program moved from discovery through candidate selection in the same amount of time.
Trevena uses its ABLE platform to discover and develop differentiated GPCR-targeted biased ligand therapeutics.
Allosteric Modulation
Given both the precise determination among variable cascade effects and complexity of interfering with large molecular weight natural ligands, allosteric modulation (i.e., action at non-native binding sites) affords a means of incorporating specificity into the regulation of GPCR activity. At the conference, Robert Lütjens, Ph.D., head of core biology at Addex Pharma , noted three key advantages to the allosteric approach.
First, given that allosteric modulation is a noncompetitive interaction, the presence of the natural ligand in the patient does not need to be overcome for efficacy to be achieved.
Second, allosteric modulators act “like dimmers,” Dr. Lütjens explained, allowing partial blocking or partial activation of particular receptors as desired. An elegant benefit of agonistic allosteric modulation is also the capacity to augment a natural response at the moment of a typical biological phenomenon; for example, GLP1 is released when food is ingested, but the duration of this response is quite short.
Positive allosteric modulation in this case “can increase a natural response at the moment it is needed in the body. Allosteric modulation thus allows us to retain the natural rhythm of the receptor activity,” Dr. Lütjens explained, adding that the third benefit was the possibility for precise selectivity, something often impossible with orthosteric (native-binding-site) approaches.
Dr. Lütjens described the company’s ProxiLite™ assay, designed to detect allosteric modulatory activity with greater sensitivity than afforded by conventional high-throughput screening techniques. “The idea is to measure receptor engagement online in a signal that occurs very proximally to the activation events. This means less interference because we’re not then dealing with the whole signaling cascade, reducing the probability of false positive and false negatives from screens, and also providing a means of informing lead optimization programs.”
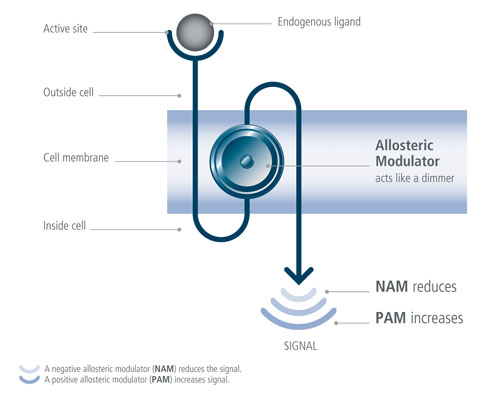
Addex Pharma’s allosteric modulator approach effects “dimmer” control over GPCR signaling, modulating intensity of activation or deactivation. Addex’ drug candidates do not bind at the same sites as do endogenous ligands (i.e., orthosterically), thus the body can retain its natural control over receptor activation, the company explains.
Accessing Structures
Given that they are highly flexible and unstable when removed from their native membranes, GPCRs have been recalcitrant to techniques commonly used for soluble drug targets such as x-ray crystallography, biophysical methods, and fragment screening. Heptares Therapeutics’ method is to introduce a small number of point mutations into the protein to “greatly increase their stability and drive them into specific and chosen conformations, either active, i.e., agonist-bound, or inactive/antagonist-bound,” explained Fiona Marshall, Ph.D., CSO. “This allows the receptors to be purified while retaining their correct folding and ligand-binding properties.”
The technology, dubbed StaR® technology (for stabilized receptor) can then be used to generate structural information, either to feed virtual screening efforts or biophysical screening techniques such as surface plasmon resonance for detailed kinetic information—methods not widely feasible with GPCRs to date and that have obvious positive implications for advancing pipelines without the attrition common in classical methods of GPCR drug discovery.
Dr. Marshall noted that Heptares has produced over 20 StaRs to 13 different receptors in recent years, and this past January reached a milestone in a collaboration with Novartis valued at $200 million plus royalties for development of an undisclosed target.
Heptares is also employing fragment screening in collaborative efforts. It is using ZoBio’s Target-Immobilized NMR Screening, or TINS platform, and Selcia’s capillary electrophoresis-based platform, to demonstrate “broad applicability of the stabilized receptors in many different paradigms,” allowing novel applications for study of membrane proteins heretofore not possible, according to Dr. Marshall. “It works very well, bridging the paradigms of structure-based discovery, which is well established for soluble drug targets, with the methods for GPCRs.”
The company’s lead candidate is targeting the A2A receptor as an oral formulation for Parkinson disease. “We were able to move from a virtual-screen hit to a candidate in about 18 months, whereas GPCR candidate development might normally take two to three years,” Dr. Marshall noted. “Our technology is making GPCR R&D timelines comparable to those for enzyme targets, which is exciting.”
In the future, the company is looking to generate co-structures with allosteric regulators to afford yet further detail about GPCR activity.
Antibody-Derived Drugs
Presenting a talk on Ablynx’ Nanobodies®, a class of antibody-derived therapeutic proteins based on single-domain antibody fragments, Hilde Revets, Ph.D., senior research fellow, acknowledged that GPCR targets have been difficult to hit with monoclonal antibodies given certain intrinsic characteristics of both GPCRs and mAbs.
Nanobody therapeutic proteins are based on the smallest functional fragment (termed VHH) of naturally occurring heavy-chain antibodies. As Dr. Revets described, “the cloned and isolated VHH domain is a stable polypeptide harboring the full antigen-binding capacity of the original heavy-chain antibody. As a result of this small size and particular three-dimensional structure, Nanobodies have the potential to access uncommon epitopes on targets, as well as cavities within molecular targets hidden or shielded from the much larger conventional antibodies.”
Dr. Revets presented work on Ablynx’ oncology candidate targeting the CXCR4 GPCR, noting that the candidate is “unlikely to have the same side-effect profile” of other similar candidates being pursued in the marketplace. Nanobodies are thus not associated with the attrition rates common for small molecule drugs, given “technological and biophysical advantages that enable them to outperform conventional antibodies” in specific metrics.
“Our technology has allowed us to quickly move forward with a number of programs in cardiovascular disease, CNS, inflammation, musculoskeletal disorders, and oncology.” Currently, there are three Nanobodies in Phase II, two in Phase I, and this year, the company intends to bring three other programs to Phase I.
Aside from a seemingly robust R&D platform, Nanobodies are also permitting Ablynx to pursue different formulations. “Nanobodies are more resistant to extremes of pH and temperature and to attack by proteases than are mAbs. Nanobodies are also characterized by solubility, so we can formulate them at high concentrations.
“These characteristics together confer the possibility of alternative modes of delivery, such as pulmonary delivery via nebulizer, and we believe the technology is robust enough also to consider oral or topical applications,” Dr. Revets said. She also reported that Nanobodies can be produced at commercial efficiency in prokaryotic systems like E. coli, as well as in eukaryotic yeast expression systems, to reduce costs as well as time lines to the clinic.
As for all drug discovery and development efforts, the basics—which, again, for GPCRs can be particularly confounding—are essential. “There is no one general strategy to follow for GPCRs,” Dr. Revets asserted. “These receptors can vary widely in their activity, so it is crucial to have solid selection tools, screening assays, biological readout, and in vivo proof-of-concept models.”