April 15, 2007 (Vol. 27, No. 8)
Advanced Tool for Purification of mAbs, Fab Fragments, and Viruses
With new upstream bioprocessing technologies continuing to increase yield and product quantities, the pressure for process improvement has fallen firmly on the downstream purification stage. The widespread use of affinity purification in large-scale downstream processing has been hampered by the absence of affinity ligands that fulfill the needs of large-scale affinity chromatography, including chemical stability, tunable affinity, high selectivity, short development times, and cost in use. The development of novel ligands that are effective for commercial-scale bioprocessing is critical for economic viability and product authenticity of future biotherapeutics.
The CaptureSelect® ligand technology offers new opportunities in the field of custom-designed ligands for commercial-scale bioprocessing of therapeutic products from complex media. The suitability of CaptureSelect technology for process-scale purification has been endorsed through collaboration between BAC (www.bac.nl) and GE Healthcare to develop media for a wide range of bioprocessing challenges.
The technology, however, is also available for customized ligands through discovery programs that can produce custom ligands for proof-of-principle testing within time frames as short as six to nine months.
Single Heavy Chain Antibodies
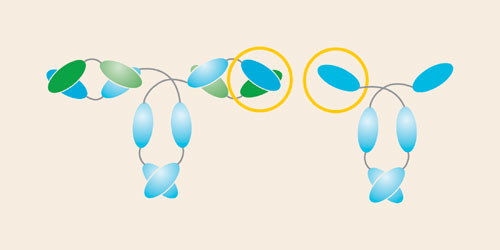
The discovery of a unique type of antibody, found only in Camelidae, has enabled the development of novel ligands for bioprocessing. The camelid antibody lacks the light chains found in all classical antibodies and as such has only one single variable domain (VHH) by which antigens are bound, and two constant domains (CH2 and CH3). Figure 1 illustrates the structure of the classical antibody and the camelid heavy chain antibody.
The single domain VHH is the smallest intact and functional antigen-binding fragment (12 kDa) derived from a fully functional immunoglobulin. Consequently, it offers improved affinity, stability, and solubility. Its small size and unique 3-D structure enables VHH fragments to recognize novel epitopes that are inaccessible to classical VH-VL pairs. In addition to being able to modify them easily, both at gene and protein level, VHH fragments have been successfully cloned and expressed in microbial systems, therefore making them ideal candidates for application as affinity ligands for biotechnological processes.
BAC has developed CaptureSelect, a technology based on camelid VHH molecules, with which a range of products for affinity purification has been developed.
Expression of the ligands in Saccharomyces cerevisiae and selection of versatile characteristics allows the fragments to be custom made for almost any biotherapeutic purification challenge.
The mRNA encoding VHH fragments is isolated from the peripheral blood lymphocytes of immunized llama through amplification using polymerase chain reaction techniques. The DNA is cloned into Saccharomyces cerevisiae creating VHH libraries, which are screened for ligands that bind to the target molecules.
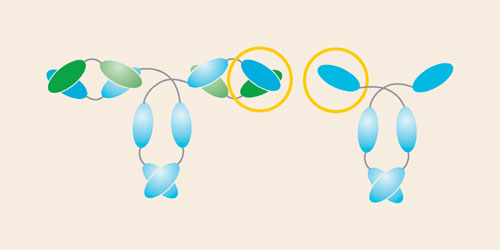
Figure 1
Production of Custom Affinity Ligands
During the screening process, specific requirements are incorporated that closely relate to the final chromatography process, such as elution conditions and stability of the ligand at certain pH values. Ligands displaying suitable chromatographic characteristics are subsequently cloned as 12 kDa fragments into a S. cerevisiae production strain, facilitating production of the affinity ligands at any scale. Expression in S. cerevisiae enables high-quality, high-titer expression within a system that is totally animal free.
The final stage of the development process is the immobilization of the ligands onto the solid support of choice. The VHH ligands can be coupled to matrices, membranes, or magnetic beads using various coupling chemistries, allowing the most suitable solid support to be selected for each application. Small batches of several ligands are produced in shake flasks and immobilized onto the chosen matrix. Product testing for chromatographic characteristics, such as binding conditions, elution conditions, and dynamic capacity, is carried out before the most suitable ligands are chosen for full-scale production.
The VHH ligand discovery process is applicable to a wide variety of target molecules. Ligands have been successfully developed for complex antigens such as bacteria and viruses; proteins, antibody fragments, and carbohydrates; and even very small molecules such as haptens, dyes, and peptide tags. The fact that large libraries of VHH ligands are screened during the discovery process makes it possible to “tune” the specificity of the ligand. Ligands can also be selected for their specificity for the format, idiotype, glycoform, or isomer of the target molecule.
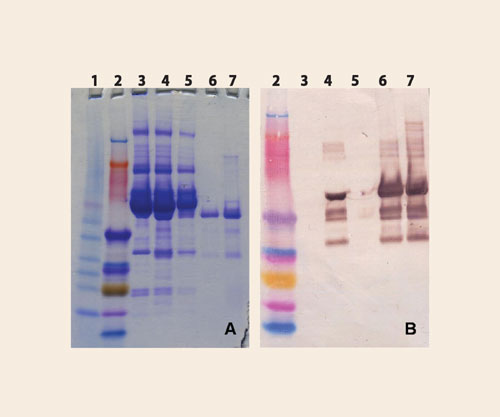
Figure 2
Ligand Discovery and Tunable Specificity
The tunable specificity of VHH ligands is exemplified by the design of affinity ligands for the separation of human IgG kappa and lambda molecules. From the library obtained from a llama immunized with polyclonal human IgM antibodies, clones producing heavy-chain antibody fragments were screened against different human IgG mAbs. Ligands that showed binding to human IgG kappa, but not to IgG lambda, were selected and produced using BAC’s S. cerevisiae production system. The ligands were coupled to NHS Sepharose 4B Fast Flow and specificity for IgG kappa was demonstrated by purification from a sample of Fab fragments derived from polyclonal human IgG (Figure 2).
The diversity of CaptureSelect technology is demonstrated through a collaboration with AMT (www.amtbv.nl) that has resulted in a simple powerful solution for the purification of the typically difficult-to-purify Adeno Associated Virus (AAV). Low recoveries are common in the purification of this virus due to a five- or six-step process, which uses a combination of gradient centrifugation, ion exchange chromatography, and heparin chromatography. In contrast, the CaptureSelect AAV ligands provide a one-step process with high selectivity to AAV subtypes 1, 2, 3, and 5, ensuring that the ligand can be used as a platform technology in the purification of different AAV subtypes (Figure 3).
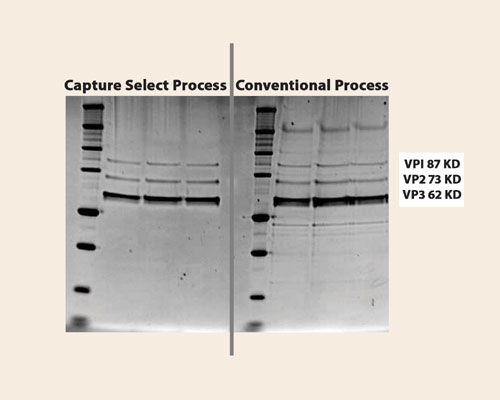
Figure 3
VHH Affinity Advantages
The CaptureSelect ranges offer the biopharmaceutical industry new tools for the purification of mAbs, Fab fragments, and viruses. In addition, the ease of custom designing these ligands provides tailored purification systems to companies for various large-scale applications such as scavenging of impurities or product isolation from complex media.
The development of affinity ligands based on the variability, specificity, and flexibility of the mammalian immune system offers huge potential to the bioprocessing industry.
The time taken from immunization to proof of principle of ligands in small-scale chromatography experiments can be as short as six to nine months, and the ease of large-scale production in well-established S. cerevisiae production strains makes VHH ligands ideal affinity tools, conferring competitive benefits in terms of reduced cost of purification, higher quality products, and increased flexibility in the purification process.
Frank Detmers is scientist (e-mail: [email protected]), Pim Hermans is director of R&D, and Mark ten Haaft is director of ligand application at BAC. Web: www.bac.nl; www.captureselect.com.