One of the fastest-growing segments of pharmaceuticals, as highlighted at the 2024 J.P. Morgan Healthcare Conference, is the antibody-drug conjugate (ADC) segment. ADCs consist of four main elements: a monoclonal antibody (mAb) designed to target a particular cancer-associated antigen; an active, anticancer cytotoxic payload; a linker that cleaves off the payload inside cancer cells; and the conjugation technology that stably attaches the linker and payload to the antibody.
The first ADC, Mylotarg, was approved in 2000, and as of October 2023, 15 additional ADCs have been approved by the FDA for treatment of a variety of cancers. Examples include Enhertu, a HER2-directed antibody and topoisomerase inhibitor conjugate; Padcev, a nectin-4-directed antibody and microtubule inhibitor conjugate; and Trodelvy, a Trop-2-directed antibody and topoisomerase inhibitor drug conjugate. In addition, Padcev and Keytruda combination therapy was approved in 2023 for first-line treatment of adult patients with locally advanced or metastatic urothelial cancer.
The exciting potential of the field is exemplified by numerous high-profile, high-value deals such as the one between Roche and Medilink Therapeutics, the one between Pfizer and Seagen, and the one between AbbVie and ImmunoGen. Also, bioconjugates are rapidly expanding beyond ADCs and oncology. In bioconjugates, the biologic needn’t be an antibody. It can be a more complex molecule. And the payload needn’t be an anticancer drug. It can be some other kind of drug.
Streamlining development
The production of bioconjugates involves several development and manufacturing steps. Accordingly, it normally requires up to seven different vendors. However, it can be accomplished by a single vendor. Such a vendor is WuXi XDC, a subsidiary of WuXi Biologics. It is a contract research, development, and manufacturing organization (CRDMO) focused on ADCs and other bioconjugate therapeutics.
“This streamlines all ADC product development activities from drug target identification to commercial production,” says Jimmy Li, PhD, the CEO of WuXi XDC. “It greatly reduces the risk of project hand-offs, intellectual property drift, and excessive transaction and audit costs.” To provide dual sourcing, a new Singaporean manufacturing base is expected to commence operations by 2026.
Utilizing highly coordinated project management, WuXi XDC reduces the integrated DNA-to-IND process for ADCs to 15 months or less, cutting the traditional timeline nearly in half. Employing the WuXiDARx technology platform, the CRDMO conjugates linker payloads at clinically validated inter-cysteine sites. This allows flexible target drug-antibody ratios (DAR) with a narrow distribution, compatibility with native IgG1 without sequence modification or glycan remodeling, compatibility with current linker-payloads, and a simple purification process.
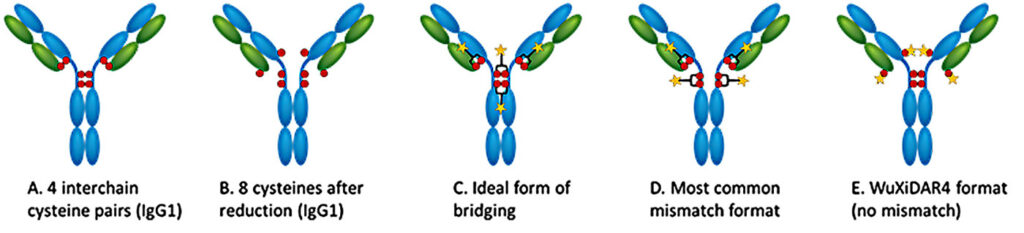
Conventional lysine or cysteine ADC conjugation technologies can result in heterogeneous products due to a wide DAR distribution. Although many site-specific conjugation technologies have addressed this issue, the improved homogeneity can come at a cost.
For example, enzymatic conjugation approaches require chromatographic purification, which adds expense, complicates the chemistry, manufacturing, and controls (CMC) process, and lowers the overall yield. Conjugation methods involving ThioMab antibodies, nonnatural amino acids, and certain enzymes require amino acid sequence modifications and are incompatible with native antibodies. Inter-cysteine bridging strategies often yield ADCs with mismatched cysteine pairs.
“Technology collaboration is one of our key pillars,” Li remarks. Recently, WuXi XDC announced a partnership with IntoCell, a Korean biotechnology company. Under the agreement, IntoCell will provide proprietary drug linker technologies using its Ortho-Hydroxy Protected Aryl Sulfate (OHPAS) linker and a new class of “OHPAS-able” camptothecins called Nexatecans.
Advancing the field
Many commercially approved ADCs are making a meaningful difference for patients suffering from life-threatening diseases. These therapeutics represent one part of the dynamically evolving bioconjugates field.
Significant advances in the field include the discovery of novel targets, innovations in the linker and payload technologies, and the introduction of new modalities, such as vaccines, imagining, immunostimulating protein-protein conjugates, or proteolysis targeting chimera (PROTAC) conjugates.
“These advancements bring the potential to better control side effects and/or increase the drug efficacy, thereby enlarging the therapeutic window,” says Christian Morello, head of bioconjugates, Lonza. According to Morello, Lonza manufactures the majority of commercialized ADCs.
The company’s recent acquisition of Synaffix brings a strong intellectual property position to create novel ADCs based on enzymatic site-selective conjugation, in combination with exclusive and secured access to state-of-the-art linker technology and an attractive selection of payloads with different modes of action.

Lonza’s development capabilities allow a smooth technology transfer of processes into GMP assets to support initiating first-in-human studies with materials and data packages. To facilitate commercialization, the company oversees the further scale-up of GMP manufacturing and ensures that complete CMC sections for biologics license applications are prepared for processes and facilities.
The integrated supply solution covers all relevant technology areas, including drug substance (proteins and drug linkers as well as bioconjugation) and drug product. “We engage with a very diverse set of bioconjugation technologies,” Morello continues. “Our track record of technology transfers allows us to transfer these, sometimes very specific, manufacturing processes to our assets. This enriches our expertise not only in a production environment, but also in the analytical development, quality assurance, and regulatory affairs of these complex molecules.”
Due to the high potency of bioconjugates, technical and safety hurdles need to be appropriately addressed. A specialized workforce with a continuous training concept and dedicated assets can overcome these challenges, especially for the steps handling cytotoxic substances. In ADC GMP manufacturing, activities that should be managed with an emphasis on safety include facility design and employee training.
Next-generation technologies
The development of ADCs requires considerable expertise across biologics, chemistry, and manufacturing. Such expertise has been cultivated by Abzena, a CRDMO that provides services spanning drug discovery, development, and manufacturing to ensure that ADCs are produced that meet the desired target product profile and perform in vivo as anticipated with the appropriate quality attributes.
“We firmly believe that good data underpin good decisions,” says Louise Duffy, PhD, the CTO of Abzena. “Our stage-appropriate and extensive analytics and bioassay services cover each stage of development.”
The company’s proprietary solutions—including EpiScreen, Composite Proteins, Composite Human Antibodies, AbZelect, AbZelectPRO, ThioBridge, and LabZient—are designed to provide the best chance of clinical and commercial success.
Complex drugs, ADCs combine the specificity of mAbs with the potency of small-molecule drugs, allowing targeted delivery of cytotoxic payloads to cancer cells. Their success relies on the careful design of key components, including the antibody, linker, and payload.
First-generation conjugation technologies have several shortcomings such as heterogeneous DAR and linker instability. Other limitations are exemplified by maleimide conjugation where there is a risk of the linker payload detaching and reattaching to free cysteines in vivo (retro-Michael reaction), reducing ADC efficacy. In the case of lysine conjugation, indiscriminate conjugation across the whole antibody can interfere with binding to the cancer antigen or the Fc receptor functions.
Duffy emphasizes that variable DAR makes it challenging to develop an efficient and robust manufacturing process. The next-generation ThioBridge conjugation technology can provide a more uniform DAR profile. Unlike maleimide conjugates, the ThioBridge conjugation technology preserves the covalent integrity of the disulfide bridges within the antibody and does not deconjugate or cross-conjugate, maintaining the structure of the ADC. The linker attachment to the antibody via its three-carbon bridge ensures stability, resulting in improved pharmacokinetic properties and, ultimately, improved efficacy.
Furthermore, ThioBridge is compatible with various payload classes and enables architectural design flexibility for linker-DAR-payload optimization. This enables more streamlined and cost-efficient production of ADCs.
Focusing on safety
For over 35 years, MilliporeSigma has provided specialized expertise to the ADC community, with 15 years as a contract testing, development, and manufacturing organization (CDMO) partnering with customers from preclinical to commercial development and manufacturing. Services across mAbs, drug linkers, and bioconjugation deliver a simplified supply chain benefiting from shared knowledge at intermediate steps.
“Our innovation and value chain accelerate development,” says Matthias Bucerius, PhD, senior vice president and general manager, Synthesis and ADC CDMO, Merck KGaA. “We are SafeBridge certified to ensure containment of potent active pharmaceutical ingredients and have produced over 100 diverse ADC constructs.”

Recent innovation includes ADCore intermediates (for expediting the synthesis of auristatin-, maytansinoid-, and pyrrolobenzodiazepine-containing payloads); ChetoSensar technology (for enhancing the solubility of ADCs with hydrophobic drug linkers); and ADC Express services (for streamlining lead candidate selection into cGMP).
Complex in composition and supply chain, each ADC construct is unique, typically contains highly cytotoxic compounds, and is the product of a multistep procedure that can result in product loss or contamination, high cost, and long execution times.
“It is critical to have proper containment and engineering controls in place with a deep understanding of critical quality attributes,” Bucerius notes. Continued improvements have been made through implementation of single-use technologies, templated processes, and end-to-end services. Over time, the manufacturing batch scale has increased significantly, from about 1 kg up to about 10 kg, requiring CDMOs to carefully consider capacity and facility optimization.
Platform technologies, such as site-specific or enzymatic conjugation strategies, are serving an increased number of assets in the ADC pipeline. The development of process and analytical technology for real-time monitoring of processes facilitates transfer to digital solutions. Data-rich development through quality-by-design principles and information technology/operational technology systems will further assist transitioning to smart manufacturing.
ADC-specific tools are also under development such as reactors or chromatography columns, and alternatives to batch production and new approaches to sustainability are being evaluated. The traditional ADC has evolved into many types of bioconjugates with novel components, requiring a comprehensive understanding of new chemistries, analytics, and critical quality attributes. “Flexibility, new technologies, and thorough technology transfer will help CDMOs adapt for increasing complexity,” Bucerius says.
Addressing challenges
AbbVie has longstanding capabilities in antibody manufacturing as well as small-molecule therapeutics. The company’s expertise in both these areas has enabled the development of highly efficient, well-controlled manufacturing processes for all clinical candidates, including ADCs.
ADCs, which represent a cutting-edge fusion of antibodies and potent cytotoxic drugs, are showing promising efficacy in targeted cancer therapy. However, ADC manufacturers must overcome several complex challenges. For example, ADC manufacturers must achieve consistent antibody-drug conjugation, optimize linker chemistry for stability and efficacy, and ensure scalability while managing costs. These challenges have prompted industry-wide innovations in automation and process optimization, as well as collaborations among industry players.
AbbVie has improved its molecule manufacturing capabilities to support the production of highly potent payloads. To better control DARs, the company has incorporated the use of engineered antibodies with superior ADC profiles. To better control conjugation, it has pioneered the use of more selective chemistry. And to better support a variety of indications, it has developed the capability to manufacture different presentations of drug products.
As ADCs become more precise and complex, CDMOs will need to upgrade their technologies and expertise. This involves using advanced methods for manufacturing, quality control, and purification.
Collaborations with technology providers and flexible manufacturing processes will facilitate adaptation to the changing demands. Adhering to strict regulations requires continuous improvement in manufacturing practices for these evolving therapeutics.


