Sponsored Content Brought to you by
The growing availability of vaccines over the last century has transformed human health. Without them, we would see a return to almost medieval conditions, where entire populations are devastated by preventable diseases. But we can’t rest on our laurels just yet – there are still improvements to be made. Reliance on old production methods and technologies, emerging strains of disease, and shortages in developing countries all continue to threaten global health. And biopharma has an essential role to play in moving this field forward.
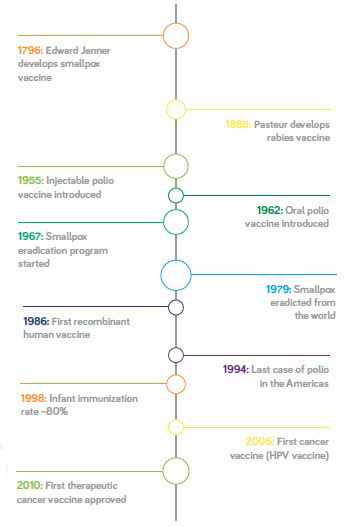
It’s widely accepted that vaccination is an effective and cost-efficient way to prevent disease, and vaccines have also led to the elimination of various conditions (see Figure 1). It has been estimated that each dollar spent on a vaccine is equivalent to 10–20 dollars that would have been spent on disease therapy, if the vaccine wasn’t available. But it is this low cost that is actually one of the challenges facing vaccine manufacturers. Because vaccines are relatively cheap compared with other biopharmaceuticals (in some cases under a dollar per dose), the cost of production is a significant part of the total cost, so there is a great deal of pressure on manufacturers. Indeed, making the business case for vaccines is becoming increasingly difficult.
Despite this, big vaccine players, such as Merck Sharp & Dohme, GlaxoSmithKline, Sanofi, and Pfizer, are committed to keeping prices low, especially in developing countries that cannot afford to pay Western prices. But a balance must be struck – if you are a for-profit business and you can’t make a profit, you can’t continue to manufacture vaccines for a longer period into the future. In general, very high volumes are needed to return a profit – think of Merck Sharp & Dohme’s HPV Gardasil vaccine, which has become part of the standard vaccination program in many Western countries; the company can turn a profit because so many doses are being sold. But not all vaccines are suited for high volumes and a number of companies have left the vaccine space completely.
Even after you have invested in developing a vaccine, continuous investment is required to ensure the vaccine remains effective, such as when pathogen strains change. Relying on old processes because you can’t make a business case for upgrades can lead to shortages in supply, which in turn threatens the vaccination rate and the herd immunity effect, with potentially huge ramifications (see Figure 2).
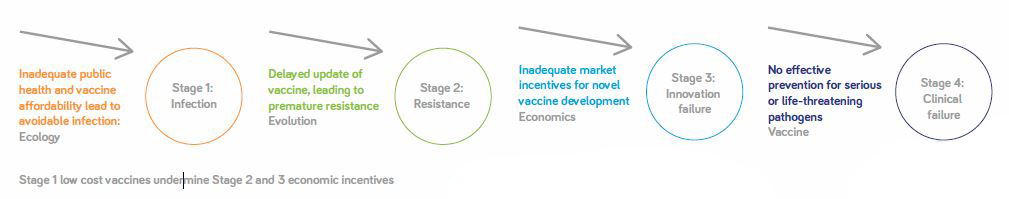
As vaccines are competing with other therapeutics in the R&D budget, which are likely to be more profitable and require less complex clinical trials, there is no easy answer to the question of how companies can create affordable vaccines and still succeed from a business point of view. Fortunately, many local manufacturers in emerging markets are stepping up and producing cheaper vaccines, particularly for pediatric use; for example, the Serum Institute of India is a big producer – actually the largest in the world by number of doses produced – as is CNBG in China. These organizations and others like them are jumping into a niche where Western corporate businesses may not always see a business. Obviously, such organizations are working with a different business model and different expectations regarding revenue and profits. Their impact on global health is not only admirable – it would not be an exaggeration to call it essential.
The support of charitable organizations is currently crucial to vaccine development – and will likely continue to be so. As income levels increase over time, some countries may need to be convinced, on a political level, to prioritize and invest in vaccines. Mutual agreement is needed between the businesses developing the vaccines and the nations in need of them to ensure prices are both affordable, and sustainable – which is no easy task!
Making It to Market
Developing a vaccine isn’t easy either. There must be no dangerous side effects (vaccines are given to healthy people who will not accept severe risk) and you need enormous clinical trials. If you want to show that you can protect against a disease, you need to give the vaccine to people that are being exposed, and you need to have a natural rate of infection that is sufficiently high to prove that the vaccine is efficacious. For example, when the new rotavirus vaccines were developed, they were tested in 50,000 children to show efficacy.
It’s important to remember that a vaccine is a very complex type of therapeutic as it must induce an immune response without creating the negative effects of the pathogen. There is a large diversity of vaccines to consider, from viruses and bacteria to polysaccharides and proteins, with a resulting downside: there are no standard platform processes for manufacture. Each vaccine has a unique production process – you essentially need to develop a new process from scratch. Yields can also be low, or very low, compared with monoclonal antibodies, which you can relatively easily produce in cell lines to quite high concentrations compared to what is currently possible for vaccines.
Another effective strategy for vaccine production is to tie development in with what is happening in the therapeutic world. We’re also seeing more technologies crossing through into the vaccine space. For example, checkpoint inhibitors, which essentially unblock the immune system, can be used in combination with vaccines for higher efficacy.
Tips for Success
The pharma companies currently succeeding in the vaccine area tend to be very large, with all the benefits of scale – established products, relatively little R&D costs, and the ability to offset low prices for countries that need them, with their own expenses.
But you don’t need to be a huge company to succeed; you can also win by using creative ideas. Many vaccines currently on the market were developed a long time ago using old technologies, and established manufacturers often don’t want to change their production technologies. For newer companies coming into this area, there’s an opportunity to develop new and innovative processes that are more cost efficient, or provide a high yield of vaccine. Companies are now looking for more advanced development and manufacturing methods, such as using virus-like particles, messenger RNA, and viral vectors. Some of these vaccines are easier to characterize and lend themselves more readily to production and purification. They can also allow manufacturers to create more homogenous products compared with older legacy vaccines. Pfizer is a good example – the company’s pneumococcus vaccine Prevnar has been a huge success thanks to an advanced conjugation vaccine platform. If you don’t come up with high value creative drugs or new production technologies or processes, you will likely have to focus on scale and volumes to be able to compete and make a profit.
Despite the challenges, it’s an exciting time to be involved in vaccine development and manufacture. As our understanding of the immune system continues to improve, so will our technologies. Vaccines are poised to be used not only for the prevention of disease, but also for treatment; there are many clinical trials now underway looking to use new technologies to kill cancer cells and treat disease that has already developed. We are entering a new era and, now more than ever, we need bright ideas, real innovation and fresh perspectives.
Günter Jagschies is Senior Director of Strategic Customer Relations, GE Healthcare, Freiburg, Germany; Mats Lundgren is Customer Applications Director, GE Healthcare, Uppsala, Sweden.








